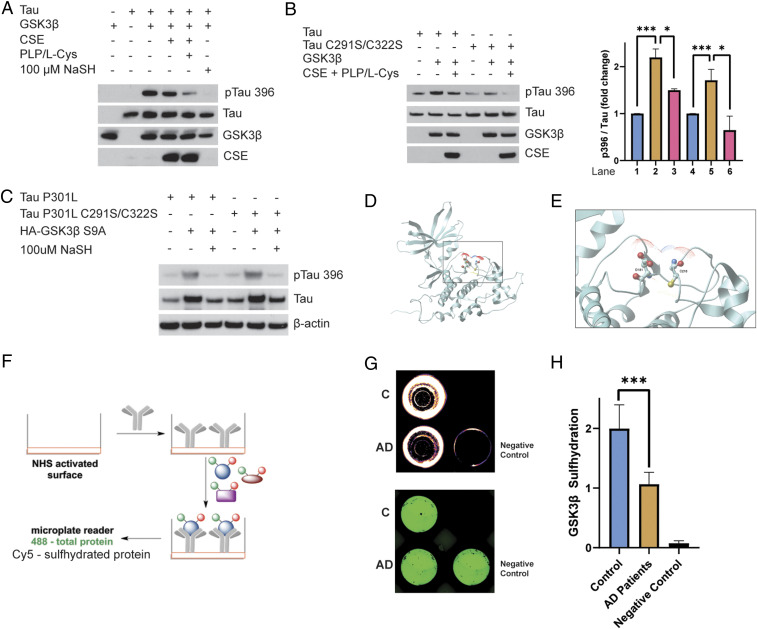
Fig. 3.
CSE and H2S inhibit phosphorylation of Tau by GSK3β. (A) Phosphorylation assays with purified Tau, GSK3β, and CSE in vitro in the presence or absence of L-cysteine (L-Cys) and PLP or treated with 100 μM NaSH. Phosphorylation of Tau was assessed by Western blotting using antibodies against phosphorylated Tau (pTau 396). Tau phosphorylation was significantly diminished when CSE, L-cysteine, and PLP were added. Addition of NaSH alone in the absence of CSE also prevented Tau phosphorylation. (B) Cysteine residues do not play a role in phosphorylation of Tau by GSK3β. Purified Tau or Tau C291S/C322S and GSK3β were incubated in the presence or absence of L-cysteine and PLP and analyzed for phosphorylation of Tau at Ser396. Western blot analysis revealed that mutation of cysteine residues Cys291 and Cys322 does not affect phosphorylation of Tau at Ser396 (n = 3, bars indicate SEM, *P < 0.05, ***P < 0.001). (C) H2S inhibits phosphorylation of P301L Tau by GSK3β. HEK293 cells were transfected with Tau P301L or Tau P301L C291S/C322S and GSK3β S9A, treated with 100 μM NaSH for 24 h, and analyzed for phosphorylation of Tau at Ser396 by Western blotting. While GSK3β phosphorylated Tau, NaSH prevented this phosphorylation. (D) Ribbon model of GSK3β (Protein Data Bank ID code 1J1B; DOI: 10.1107/S090744490302938x). Intercept: (E) thiolate side chain of Cys218 (ball-and-stick model) that we found to be sulfhydrated is already in close proximity to Asp181 in the active site of GSK3β, so the presence of an additional sulfur atom will inevitably alter the conformation of the active site, which would inhibit its kinase activity. Oxygen atoms are shown in red, sulfur in yellow, and nitrogen in blue. Dots around the atoms represent expected water surface accessibility. (F) Schematic representation of the antibody array-like approach to study sulfhydration status of GSK3β in AD brains. Anti-GSK3β antibody was immobilized on a 96-well plate with N-hydroxysuccinimide–activated surface. Brain cortical lysates from normal and AD postmortem tissues were added to the wells to allow recognition of GSK3β from lysates by the antibody. The bound protein was labeled with NBF (green) for a total load and with Cy5 (red) for sulfhydration, and the ratio of the two signals was measured to yield sulfhydration levels. As a negative control, 488-labeled albumin (instead of antibody) was used to block the available surface and then incubated with control lysates. (G) Readout from a representative experiment showing decreased sulfhydration (red) in cortex of AD patients, while the negative control shows no signals. The plate was recorded on a Typhoon FL9500 at 488 nm (NBF fluorescence signal in green represents total load) and 635 nm (Cy5 signal in red represents sulfhydration). (H) Quantitation of H (n = 4, bars indicate SEM, ***P < 0.001).