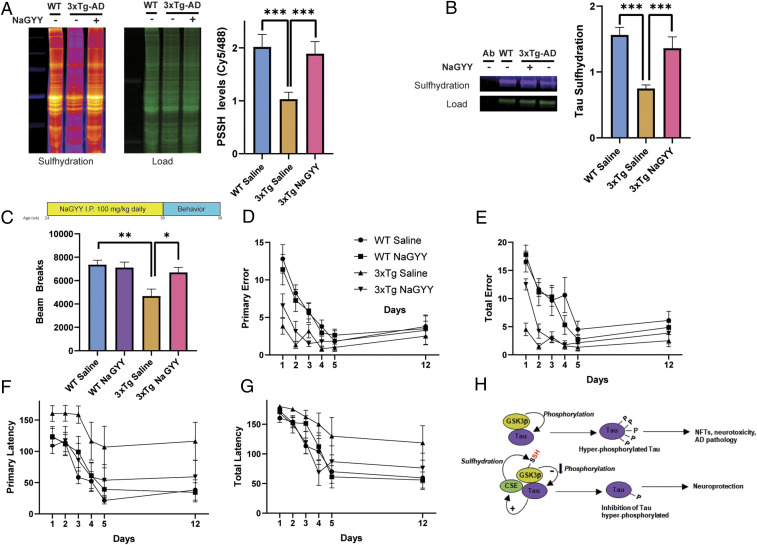
Fig. 4.
The H2S donor NaGYY ameliorates AD symptoms. (A) Overall sulfhydration is decreased in the hippocampus of 3xTg-AD mice, which is rescued by NaGYY treatment in 3xTg-AD mice as revealed by the dimedone switch method (n = 3, bars indicate SEM, ***P < 0.001). (B) Sulfhydration of Tau is decreased in the hippocampus of 3xTg-AD mice as revealed by immunoprecipitation assays in combination with the dimedone switch assay. Treatment with NaGYY rescues sulfhydration of Tau. (C) Treatment regimen for 3xTg-AD mice with the H2S donor NaGYY. Mice were treated at 6 mo with 100 mg/kg NaGYY by intraperitoneal injection daily for 12 wk, and behavioral analyses were conducted at 9 mo. The open-field test revealed significant deficits in locomotor activity in the male 3xTg-AD mice, which were rescued by NaGYY (n = 6 to 10, bars indicate SEM, **P < 0.01 and *P < 0.05). (D–G) NaGYY partially rescues memory deficits in the 3xTg-AD mice. These mice do not exhibit significant differences in primary error and total error in the Barnes maze test (D and E). NaGYY treatment partially rescues primary and total latency (F and G; n = 6 to 10, bars indicate SEM, *P < 0.05 for comparison between primary latency of 3xTg-AD saline and 3xTg-AD NaGYY by one-way ANOVA followed by a post hoc Tukey test). (H) Model depicting a possible mode of neuroprotection afforded by H2S. GSK3β (yellow-ochre) binds Tau (purple) and phosphorylates it (marked as “P”), which leads to the formation of NFTs and AD pathology in the 3xTgAD mice. H2S produced by CSE (green) sulfhydrates GSK3β (“SH” in red text) and inhibits phosphorylation of Tau. Tau binds to CSE and enhances its activity (arrow with a plus sign), forming part of a virtuous cycle that decreases Tau phosphorylation and confers neuroprotection.