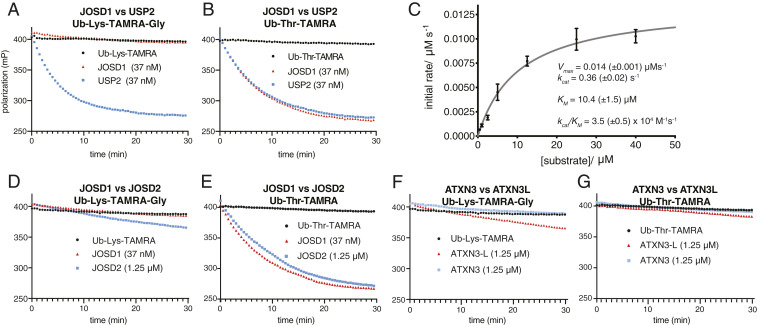
Fig. 5.
Characterization of MJD DUB esterase activity by FP-based assay toward model amino acid substrates. (A) Ub-Lys-TAMRA-Gly tested at 250 nM. The USP class DUB USP2 demonstrates efficient isopeptidase activity toward Ub-Lys-TAMRA-Gly whereas JOSD1 isopeptidase activity is undetectable. (B) JOSD1 demonstrates efficient esterase activity toward Ub-Thr-TAMRA with comparable kinetics to that of USP2. (C) Steady-state Michaelis–Menten analysis for JOSD1 esterase activity toward Ub-Thr-TAMRA (JOSD1 assay concentration was 37 nM). (D) Negligible JOSD2 isopeptidase activity is also observed toward Ub-Lys-TAMRA-Gly (note JOSD2 concentration is 1.25 μM). (E) JOSD2 is a less efficient esterase than JOSD1 toward Ub-Thr-TAMRA, but cleavage kinetics are comparable when JOSD2 concentration is increased ∼30-fold relative to JOSD1. (F) ATXN3 esterase activity is negligible toward the synthetic Ub-Lys-TAMRA-Gly substrate whereas ATXN3L exhibits weak isopeptidase activity. (G) Both ATXN3 and ATXN3L exhibit negligible esterase activity toward Ub-Thr-TAMRA.