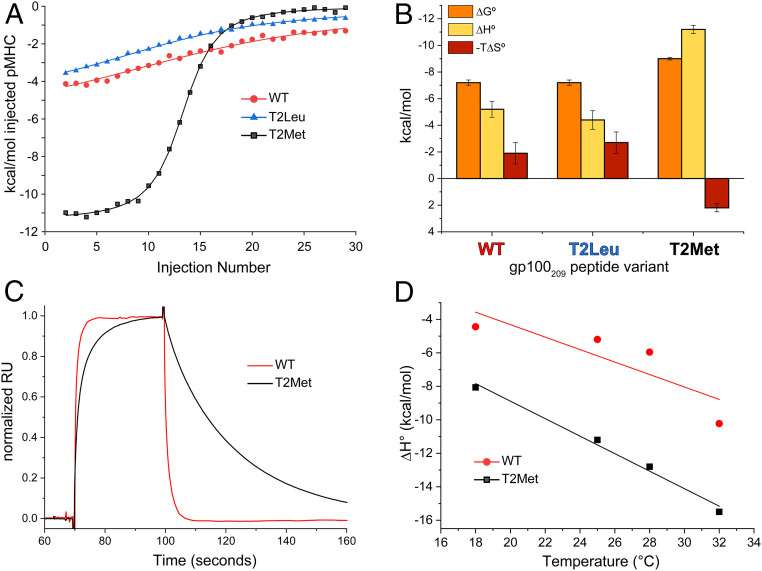
Fig. 2.
The T4H2 TCR recognizes the T2Met peptide/HLA-A2 complex with a distinct thermodynamic and kinetic profile. (A) Calorimetric titrations of peptide/HLA-A2 complexes with the T4H2 TCR. The WT and T2Leu complexes are recognized similarly, but recognition of T2Met is strikingly more endothermic. Multiple datasets were analyzed globally with shared stoichiometries and local thermodynamics, facilitating accurate assessments across a range of c values as described in Methods. (B) Breakdown of binding thermodynamics for the data in A. Compared to WT and T2Leu, T2Met is recognized with a much more favorable enthalpy change and an unfavorable entropy change. Values are the average and SDs from the indicated number of titrations in SI Appendix, Table S3. (C) T4H2 binds the T2Met complex with slower association and dissociation rates compared to the WT complex. Data are from the injection of 18 μM of peptide/HLA-A2 complex over the same TCR sensor surface, normalized to the maximum response point (prior to injection spikes) to facilitate comparison of kinetic phases. The slower association and dissociation phases for the T2Met complex are evident (SI Appendix, Fig. S2B). (D) Changes in heat capacity for T4H2 recognition of the WT and T2Met peptides calculated from the binding ΔH° measured at different temperatures.