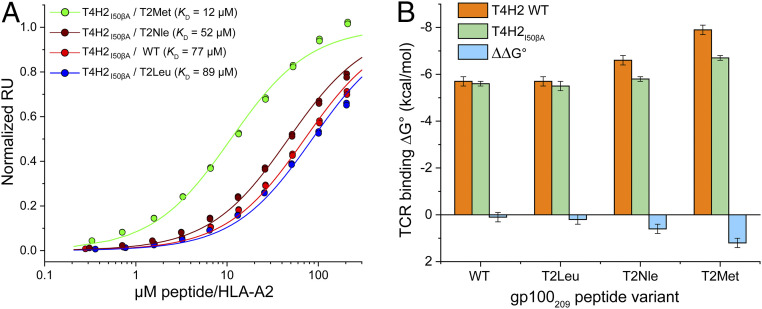
Fig. 5.
Peptide-dependent consequences of a mutation in the T4H2 CDR2β loop confirms the structural variances in the T4H2 structures. (A) SPR titrations for the T4H2 TCR with the Ile50β→Ala mutation binding the WT, T2Nle, T2Leu, and T2Met peptide/HLA-A2 complexes. Data points show representative single titrations with duplicate injections. (B) Comparison of binding free energy changes with the WT T4H2 TCR and the Ile50β→Ala mutant. The effect of the mutation, ∼20-Å distant from the p2 residue, is negligible for recognition of the WT peptide, but increases upon p2 anchor modification, to a maximum amount observed with the T2Met peptide. Values and error bars are from SI Appendix, Table S2, propagated from the average and SDs of the KD values.