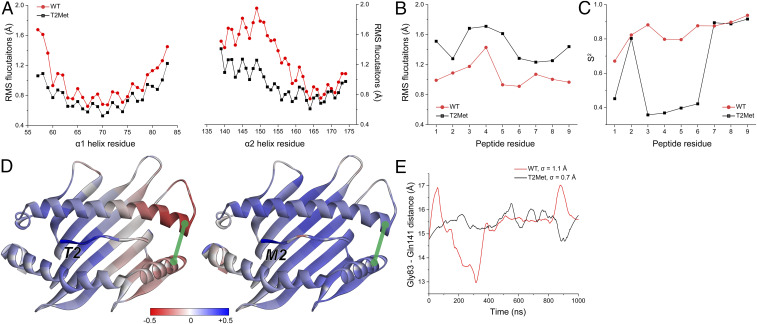
Fig. 6.
Anchor modification alters the motional properties of the unbound peptide/HLA-A2 complex. (A) The RMS fluctuations for Cα atoms of the HLA-A2 α1 and α2 helix when the WT or T2Met peptide is bound. A clear difference is apparent in the C-terminal short arm of the α2 helix. (B) The RMS fluctuations for Cα atoms of the peptides bound to HLA-A2. T2Met peptide fluctuates more than the WT peptide in the binding groove. (C) Order parameters (S2), calculated from vectors defined between the α-carbons and β-carbons of each residue. Consistent with the fluctuation data, the WT peptide is more ordered in the binding groove. (D) Correlations between the p2 Cα atom and the Cα atoms of the rest of the WT and T2Met peptide/HLA-A2 complexes. Red/blue colors indicate anticorrelated/correlated motion according to the indicated scale. Replacing Thr with Met at p2 shifts motion in the peptide binding groove from anticorrelated to correlated. Green line represents the distance between Gly83 and Gln141. (E) Variance in Gly83–Gln141 distance over the course of the simulations when the WT or T2Met peptides are bound. The WT complex displays more breathing between the helices.