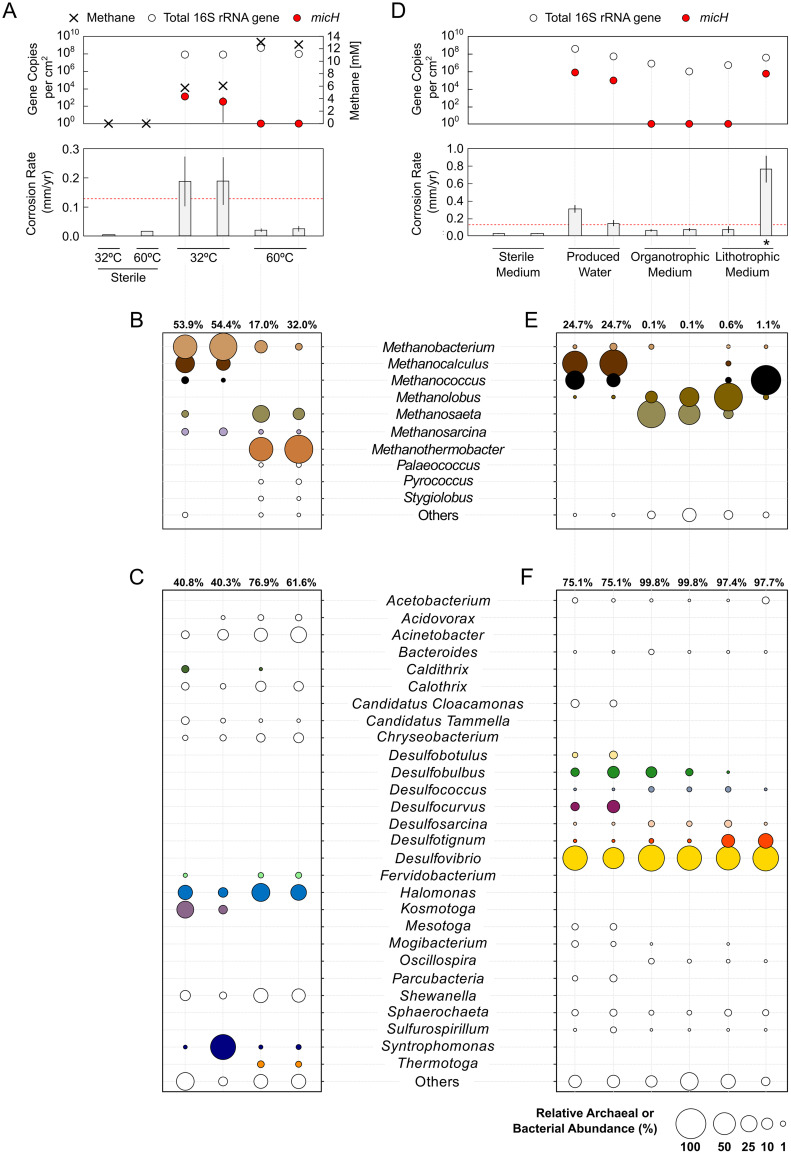
FIG 2.
Growth of steel-attached oil field biofilms in bottle tests. (A to C) Tests with sulfate-free anoxic produced water obtained from the West African oil field and incubated under mesophilic (32°C) and thermophilic (60°C) conditions. (D to F) Tests with sulfate-amended produced water, as well as synthetic produced water medium (plus sulfate) in the presence or absence of propionate and acetate (organotrophic and lithotrophic conditions, respectively). (A and D) Averaged weight loss corrosion rates (CR), total 16S rRNA gene (archaeal and bacterial) quantification, and gene copy numbers of the proposed archaeal MIC biomarker micH. A threshold denoting technically relevant, high corrosion rates (≥0.13 mm Fe0 · yr−1) is indicated by the dashed red line. Methane formation is depicted as the final concentration in headspace after 3 months (A). Archaeal (B and E) and bacterial (C and F) community composition was assessed by 16S rRNA gene sequencing of DNA extracted from biofilms grown on carbon steel coupons. Numbers above the abundance plots indicate the percentage of total sequencing reads. Archaeal sequences accounted for 0.1 to 54.4% and bacterial sequences for 40.3 to 99.8% of the total sequencing reads. Genera with abundances of <1% in either the bacterial or archaeal fraction of all biofilm samples were merged under “Others.” The enrichment culture that was used for a subsequent lithotrophic corrosion kettle test is indicted by an asterisk. The error bars depict the standard deviation of technical replicates (n = 3) for weight loss corrosion rates and micH qPCR analysis.