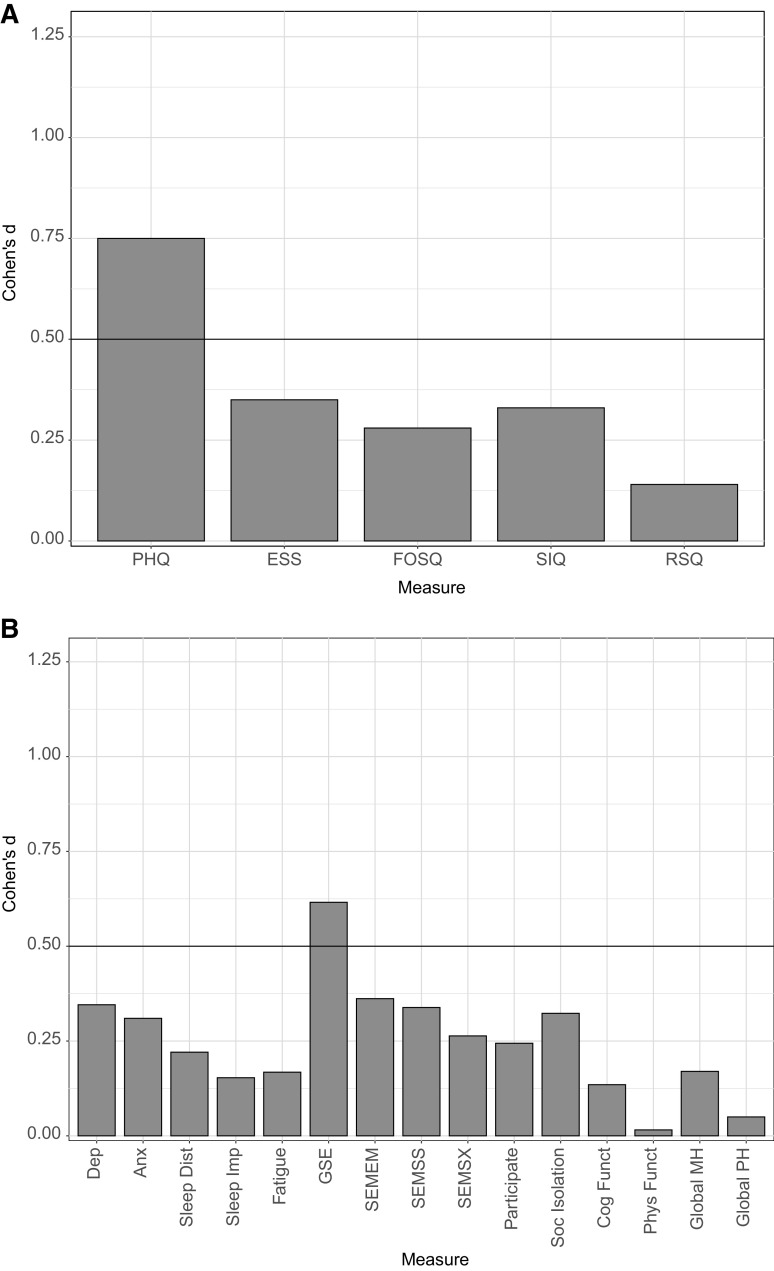
Figure 2. Within-subject effect sizes by clinical measures for total sample.
(A) The within-subject effect size (Cohen’s d) from baseline to posttreatment for each of the following clinical measures: Patient Health Questionnaire (PHQ), Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ), Sleep Inertia Questionnaire (SIQ), and Restorative Sleep Questionnaire (RSQ). (B) The within-subject effect size (Cohen’s d) from baseline to posttreatment for each of the following Patient-Reported Outcomes Measurement Information Systems measures: Depression (Dep), Anxiety (Anx), Sleep Disturbance (Sleep Dist), Sleep-Related Impairment (Sleep Imp), Fatigue, General Self-Efficacy (GSE), Self-Efficacy for Managing Emotions (SEMEM), Self-Efficacy for Managing Social Interactions (SEMSS), Self-Efficacy for Managing Symptoms (SEMSX), Ability to Participate in Social Roles and Activities (Participate), Social Isolation (Soc Isolation), Cognitive Functioning (Cog Funct), Physical Functioning (Phys Funct), Global Mental Health (Global MH), and Global Physical Health (Global PH). An effect size of d > 0.5 was the prespecified benchmark for a minimal clinically important difference.