Abstract
Purpose:
We evaluated demographic and clinical characteristics associated with participation in a clinical trial testing the efficacy of an online tool to support breast cancer risk communication and decision support for risk mitigation to determine the generalizability of trial results.
Methods:
Eligible women were members of Kaiser Permanente Washington aged 40-69 years with a recent normal screening mammogram, heterogeneously or extremely dense breasts and a calculated risk of > 1.67% based on the Breast Cancer Surveillance Consortium 5-year breast cancer risk model. Trial outcomes were chemoprevention and breast magnetic resonance imaging by 12-months post-baseline. Women were recruited via mail with phone follow-up using plain language materials notifying them of their density status and higher than average breast cancer risk. Multivariable logistic regression calculated independent odds ratios (ORs) for associations between demographic and clinical characteristics with trial participation.
Results:
Of 2,569 eligible women contacted, 995 (38.7%) participated. Women with some college (OR = 1.99, 95% confidence interval [CI] 1.34-2.96) or college degree (OR = 3.35, 95% CI 2.29-4.90) were more likely to participate than high school-educated women. Race/ethnicity also was associated with participation (African-American OR = 0.50, 95% CI 0.29-0.87; Asian OR = 0.22, 95% CI 0.12-0.41). Multivariate adjusted ORs for family history of breast/ovarian cancer were not associated with trial participation.
Discussion:
Use of plain language and potential access to a website providing personal breast cancer risk information and education were insufficient in achieving representative participation in a breast cancer prevention trial. Additional methods of targeting and tailoring, potentially facilitated by clinical and community outreach, are needed to facilitate equitable engagement for all women.
Keywords: breast cancer, decision-making, risk
INTRODUCTION
Breast density is one of the strongest risk factors for breast cancer, after age and family history, with dense breast tissue conferring a 3- to 6-fold increased risk of breast cancer.1-5 Most women, however, are not aware of their breast density status or that breast density is an independent breast cancer risk factor.6 In an effort to increase women’s awareness of breast density, 37 states have enacted legislation requiring breast density notification after mammography,7 and in 2019 federal law now requires that the Food and Drug Administration develop reporting language about breast density.8 To some extent, notification laws have led to improved knowledge of breast density status and motivating women towards clinical follow-up relative to states without notification.6 However, results are mixed as to whether density legislation leads to appropriate clinical translation, in terms of breast cancer risk assessment and supplemental screening,9 or serves as a source of confusion for both women and clinicians.10-13
The addition of breast density in breast cancer risk prediction tools more accurately discriminates cancer risk14,15 and provides an opportunity to share with women a highly relevant and underappreciated risk factor. Hence, effective interventions that support women in breast health with risk assessment, clinical education, targeted screening recommendations, and shared decision-making should be evaluated for alignment within clinical care. Haas et al. randomized 459 women with recent normal mammograms to receive either a brief video personalized to a woman’s density (high vs low) and breast cancer risk status (high vs average) or usual care (ie, a form letter).16 The personal video significantly improved women’s knowledge of their density status and breast cancer risk compared to usual care. Further, women who viewed the video were more likely to discuss their mammogram results with their primary care provider. These promising results are tempered by the fact that the predominantly white and highly educated participants in this trial were not representative of the underlying population of women at risk for breast cancer. Further, with only 9% of participants having clinically elevated breast cancer risk,17,18 those most likely to benefit from the intervention were also underrepresented in the trial.
In this study, we describe participation in a randomized trial that targeted women at clinically elevated breast cancer risk based on their breast density and additional breast cancer risk factors. The purpose of the randomized trial was to test a web-based breast cancer risk communication and decision-making tool compared with usual care. We describe overall participation rates in the trial and variation in participation based on demographic and clinical characteristics.
METHODS
Study Population and Setting
Eligible women were aged 40-69 years with a recent normal mammogram and members of Kaiser Permanente Washington, an integrated care delivery system. At the time of the research study (2017-2018), state law did not mandate reporting of breast density. However, as a practice, Kaiser Permanente Washington included the Breast Imaging Reporting and Data System (BI-RADS)19 breast density assessments in the radiology mammography report, which was available in the online patient portal.
Women self-reported breast cancer risk factors at the time of a screening mammogram, including age, race/ethnicity, first-degree family history of breast cancer, history of breast procedures, and other risk factors. BI-RADS breast density was determined by the reading radiologist of the mammogram.
Five-year breast cancer risk was calculated using the Breast Cancer Surveillance Consortium (BCSC) Five Year Risk Calculator.17 To be eligible for the trial, women had to be at elevated risk based on a combination of their BCSC risk and their BI-RADS breast density assessment. Thus, women were eligible for the study if they had either: 1) an intermediate 5-year risk of invasive breast cancer (1.67%-2.49%) and extremely dense breasts; or 2) a high 5-year cancer risk (≥ 2.50%) and either heterogeneously dense or extremely dense breasts. Primary clinical outcomes of the trial were chemoprevention prescriptions and receipt of breast magnetic resonance imaging by 12 months after the baseline interview. Additional outcomes were self-reported cancer-related distress, clinician conversations about chemoprevention and breast MRI, and mammography maintenance. Notably, the study outcomes were not noted in our recruitment materials.
We excluded women with a personal history of lobular carcinoma in situ, any cancer diagnosis excluding non-melanoma skin cancer, and a previous referral for cancer genetic counseling and/or prior genetic testing. Additional details of the trial methods are available.20,21
Recruitment
Study recruitment materials (see Appendix at www.thepermanentejournal.org/files/2020/19.205supp.pdf), including letters and telephone scripts, developed by the study team and edited by a plain language consultant at Kaiser Permanente Washington Health Research Institute.22 Materials were drafted with a literacy level at the 6th grade. Women were invited to participate in a research study “because their recent mammogram showed that (they) had dense breast tissue. Having dense breast tissue, along with other risk factors (such as age, family history, or prior breast biopsy) means (their) risk of developing breast cancer is higher than average for a woman of (their) age and race.” Women who were assigned to the intervention were told they would “learn about breast density, their personal breast cancer risk, options for screening and prevention and steps they can take to manage their risk.” (see Appendix)
From February 2017 to May 2018, all eligible participants were mailed a study recruitment letter within 6 months of their most recent normal mammogram (median = 4.5 months). A survey team member followed-up by phone within a few days to assess eligibility and willingness to participate. Women were contacted up to 10 times by telephone. Eligible women who enrolled in the trial completed a baseline interview by telephone. Women were then emailed a link to the study website where they provided informed consent. Consented participants were then directed to either the intervention website (intervention group) or general content about breast health (control group). The intervention group received personalized 5- and 10-year breast cancer risk estimates online; information about chemoprevention and breast magnetic resonance imaging; and were able to complete a values clarification and question prompt list to share with their primary care provider. All participants were encouraged to talk to their primary care provider about their breast cancer risk.
Statistical Analysis
We describe trial enrollment and reasons for non-participation in a Consolidated Standards of Reporting Trials diagram (Figure 1). Participants included women who completed a baseline interview and provided inormed consent. Non-participants included all other eligible women, who did not consent to participate in the trial. Women ineligible at the time of telephone contact were excluded from the study population. We also examined recruitment yield from each step of our enrollment procedures.
Figure 1.
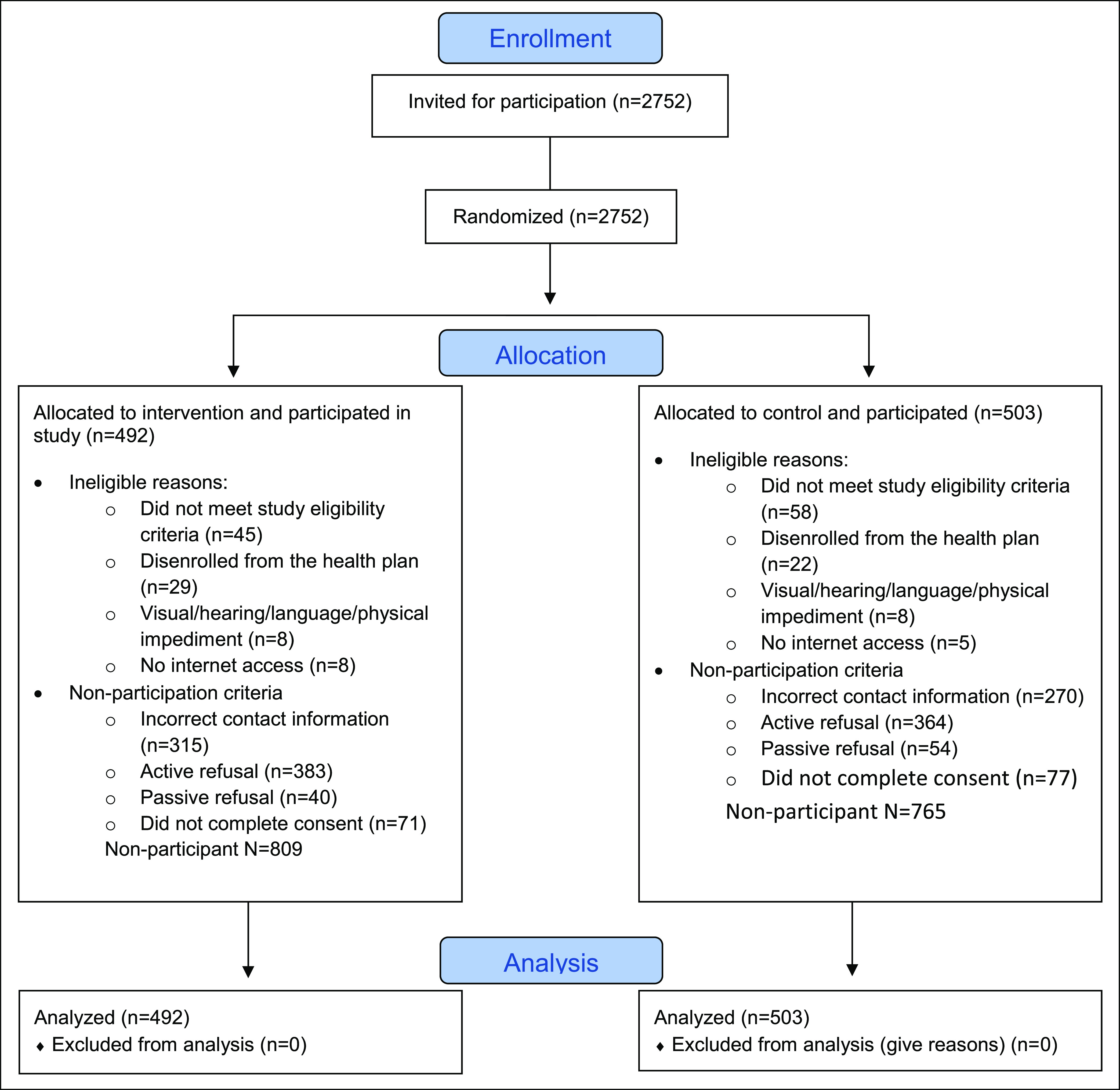
Consort diagram of patient participation in ENGAGED 2 study 2017-2018.
Patient characteristics were self-reported at the time of the most recent mammogram to assess relationship with participation. Women’s current addresses were linked to census data to impute household income. We calculated descriptive frequencies of baseline characteristics by participation status. We used multivariable logistic regression to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for demographic and clinical characteristics independently associated with trial participation. Given that the BCSC model accounts only for the presence of an affected first-degree relative and not specific aspects of family history that are clinically relevant, we also examined specific components of cancer family history available from self-reported questionnaires in a separate multivariate model. Analysis was conducted using Stata version 15 by StatCorp, College Station, TX.23
RESULTS
We contacted 2,569 eligible women with 995 (38.7%) who participated, with similar proportions by intervention and control group (Figure 1).
We examined the number of contacts required for study enrollment (data not shown). Among women who enrolled in the study, 71% enrolled by the 4th call attempt (12.4% in call 1, 26.4% in call 2, 19.2% in call 3, and 12.9% in call 4). Similarly, among women who actively refused, 68% refused by 4th telephone attempt (30.2% in call 1, 16.6% in call 2, 11.2% in call 3, and 10.2% in call 4). For each phone attempt, the proportion of women who enrolled in the study increased from 5.2% at call 1 to 13.3% enrolled in call 2 (highest) and then diminished slowly to 5-7% enrollment by the 6th call attempt.
Women who participated were more likely to be age 60 or older, White, and have some college education or college degree compared with non-participants (Table 1). Further, a higher proportion of women who participated had BCSC 5-year risk > 2.5% and heterogeneously dense breasts. Self-report of detailed family history of breast and ovarian cancer was similar among participants and non-participants, across measures of cancer type, number of relatives, and age at diagnosis (Table 2). Among participants, 48.2% reported a first degree relative with breast cancer; and 9.6% reported any family history of ovarian cancer (Table 2). Non-participants reported similar prevalence (46.3% and 9.9%, respectively).
Table 1.
Characteristics of eligible participants by enrollment and consent in the ENGAGED 2 study in Kaiser Permanente Washington women members, 2017-2018
| Non-Participant | Participant | |||
|---|---|---|---|---|
| Characteristic | No. | (%) | No. | (%) |
| Number of women (row%) | 1,574 (61.3) | 995 (38.7) | ||
| Age at baseline | ||||
| 40-49 | 47 | 3.0 | 19 | 1.9 |
| 50-59 | 532 | 33.8 | 280 | 28.1 |
| 60-69 | 994 | 63.2 | 696 | 70.0 |
| Mean age (SD) | 60.8 (5.5) | 61.9 (5.1) | ||
| Race/ethnicity | ||||
| White, non-Hispanic | 1,385 | 88.0 | 943 | 94.8 |
| Asian/Pacific Islander | 96 | 6.1 | 14 | 1.4 |
| Black, non-Hispanic | 56 | 3.6 | 19 | 1.9 |
| Hispanic | 33 | 2.1 | 16 | 1.6 |
| Mixed or other | 4 | 0.3 | 3 | 0.3 |
| Education | ||||
| HS/GED or less | 147 | 9.3 | 38 | 3.8 |
| Some college | 443 | 28.1 | 218 | 21.9 |
| College graduate | 919 | 58.4 | 722 | 72.6 |
| Missing | 65 | 4.1 | 17 | 1.7 |
| BCSCa risk level | ||||
| 1.67-2.49 | 502 | 31.9 | 250 | 25.1 |
| > 2.50 | 1,072 | 68.1 | 745 | 74.9 |
| First degree family history of breast cancer | ||||
| No | 763 | 48.5 | 480 | 48.2 |
| Yes | 657 | 41.7 | 447 | 44.9 |
| Unknown | 154 | 9.8 | 68 | 6.8 |
| Prior breast biopsy | ||||
| No | 783 | 49.8 | 486 | 48.8 |
| Yes | 699 | 44.4 | 452 | 45.4 |
| Unknown | 92 | 5.8 | 57 | 5.7 |
| Median family income | ||||
| < $70,000 | 366 | 23.3 | 221 | 22.2 |
| $70,000-$89,999 | 334 | 21.2 | 227 | 22.8 |
| $90,000-$109,999 | 332 | 21.1 | 239 | 24.0 |
| $110,000-$129,999 | 216 | 13.7 | 128 | 12.9 |
| > $130,000 | 233 | 14.8 | 130 | 13.1 |
| Unknown | 93 | 5.9 | 50 | 5.0 |
| Menopausal status | ||||
| Postmenopause | 165 | 10.5 | 75 | 7.5 |
| Premenopause | 1,409 | 89.5 | 920 | 92.5 |
| Breast density | ||||
| Heterogeneously dense | 737 | 46.8 | 554 | 55.7 |
| Dense | 837 | 53.2 | 441 | 44.3 |
a Five-year BCSC risk based on https://tools.bcsc-scc.org/BC5yearRisk/intro.htm.
HS/GED = high school/General Education Development certificate,
Table 2.
Detailed family history of breast and ovarian cancer among participants by enrollment and consent
| Non-Participant | Participant | |||
|---|---|---|---|---|
| Characteristic | No. | (%) | No. | (%) |
| Number women with known family history data | 1,420 | 927 | ||
| Breast cancer | ||||
| FDR | 657 | 46.3 | 447 | 48.2 |
| FDR age < 50 | 241 | 17.0 | 125 | 13.5 |
| FDR, bilateral | 105 | 7.4 | 49 | 5.3 |
| 2+ relatives ages < 50 | 81 | 5.7 | 31 | 3.3 |
| 3+ relatives | 32 | 2.3 | 18 | 1.9 |
| Any male relative | 11 | 0.8 | 1 | 0.1 |
| Any other relative (aunt, grandmother) | 459 | 32.3 | 328 | 35.4 |
| Ovarian cancer | ||||
| Any family history | 141 | 9.9 | 89 | 9.6 |
| 2+ relatives on same side | 18 | 1.3 | 9 | 1.0 |
| FDR with breast AND ovarian cancer | 48 | 3.4 | 18 | 1.9 |
| Ashkenazi Jewish relative with breast OR ovarian cancer | 37 | 2.6 | 32 | 3.5 |
| One relative with breast and 1 relative with ovarian cancer on same side of family | 75 | 5.3 | 38 | 4.1 |
FDR = first degree relative.
In multivariate models, study participation was independently associated with age, race/ethnicity, and education (Table 3). For every 1-year increase in age, women were 4% more likely to participate in the trial (OR = 1.04, 95% CI 1.02-1.06). Women who identified as Black (OR = 0.50, 95% CI 0.29-0.87) or Asian/Pacific Islander (OR = 0.22, 95% CI 0.12-0.41) were significantly less likely to participate than White women. Women with some college (OR = 1.99, 95% CI 1.34-2.96) or a college degree (OR = 3.35, 95% CI 2.29-4.90) were more likely to participate than women with a high school education. No other factors were significantly associated with study participation in multivariate analysis, including menopausal status, breast density, prior biopsy, income or BCSC 5-year breast cancer risk.
Table 3.
Univariate and multivariate ORs of participant characteristics associated with enrollment in the ENGAGED 2 study
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a |
|---|---|---|
| Age (continuous) | 1.04 (1.02-1.05) | 1.04 (1.02-1.06) |
| Age group | ||
| 40-49 | 1.0 | N/A |
| 50-59 | 1.30 (0.75-2.26) | |
| 60-69 | 1.73 (1.01-2.97) | |
| Race | ||
| White, non-Hispanic | 1.0 | 1.0 |
| Black, non-Hispanic | 0.50 (0.29-0.84) | 0.50 (0.29-0.87) |
| Hispanic | 0.71 (0.39-1.30) | 0.77 (0.41-1.45) |
| Asian/Pacific Islander | 0.21 (0.12-0.38) | 0.22 (0.12-0.41) |
| Mixed/other | 1.10 (0.25-4.93) | 0.96 (0.21-4.40) |
| Education | ||
| HS/GED or less | 1.0 | 1.0 |
| Some college | 1.90 (1.29-2.82) | 1.99 (1.34-2.96) |
| College graduate | 3.04 (2.10-4.40) | 3.35 (2.29-4.90) |
| Missing | 1.01 (0.53-1.92) | 1.29 (0.67-2.48) |
| Menopausal status | ||
| Postmenopausal | 1.0 | 1.0 |
| Premenopausal | 0.70 (0.52-0.93) | 1.03 (0.73-1.47) |
| Breast density | ||
| Heterogeneously dense | 1.0 | 1.0 |
| Extremely dense | 0.70 (0.60-0.82) | 0.86 (0.68-1.07) |
| Family history | ||
| No | 1.0 | 1.0 |
| Yes | 1.08 (0.92-1.28) | 1.20 (0.94-1.53) |
| Unknown | 0.70 (0.52-0.95) | 0.74 (0.54-1.03) |
| Prior biopsy result | ||
| No | 1.0 | 1.0 |
| Yes | 1.04 (0.88-1.23) | 1.18 (0.93-1.50) |
| Unknown | 1.00 (0.70-1.42) | 1.18 (0.81-1.73) |
| Income | ||
| < $70,000 | 1.0 | 1.0 |
| $70,000 to < $90,000 | 1.12 (0.89-1.43) | 1.06 (0.83-1.36) |
| $90,000 to < $110,000 | 1.19 (0.94-1.51) | 1.13 (0.88-1.45) |
| $110,000 to < $130,000 | 0.98 (0.75-1.29) | 0.90 (0.67-1.21) |
| $130,000+ | 0.92 (0.70-1.21) | 0.91 (0.67-1.23) |
| Unknown | 0.89 (0.61-1.30) | 0.86 (0.58-1.28) |
| Five-year risk | ||
| 1.67-2.49 | 1.0 | 1.0 |
| > 2.50 | 1.40 (1.17-1.67) | 0.78 (0.56-1.09) |
a Adjusted for age (continuous), race, family history, biopsy history, income, 5-year risk, facility, education, menopausal status, and density.
Women’s self-reported family history of breast or ovarian cancer did not influence their participation in the trial, except among two key family features (Table 4). Women with a first-degree relative with bilateral breast cancer (OR = 0.69, 95% CI 0.48-0.99) or with 2 or more first degree relatives with breast cancer diagnosed under age 50 (OR = 0.63, 95% CI 0.41-0.97) were less likely to participate than women without this family history, adjusted for age, race/ethnicity, mammographic facility, and education.
TABLE 4.
Multivariate adjusted ORs of ENGAGED 2 participation compared to non-participation by breast and ovarian cancer family history characteristics
| Characteristic | Adjusted ORa (95% CI) |
|---|---|
| Breast cancer | |
| First degree relative | 1.09 (0.92-1.30) |
| First degree relative age < 50 | 0.82 (0.64-1.04) |
| First degree relative, bilateral | 0.69 (0.48-0.99) |
| Two or more relatives ages < 50 | 0.63 (0.41-0.97) |
| Three or more relatives | 1.07 (0.57-2.02) |
| Any male relative | 0.26 (0.06-1.17) |
| Any other relative (aunt, grandmother) | 1.08 (0.90-1.30) |
| Ovarian cancer | |
| Any family history | 0.92 (0.69-1.22) |
| Two or more relatives on same side | 0.97 (0.43-2.21) |
| Breast or ovarian cancer | |
| First degree relative with breast AND ovarian cancer | 0.66 (0.37-1.17) |
| Ashkenazi Jewish relative with breast OR ovarian cancer | 1.05 (0.64-1.71) |
| One relative with breast and 1 relative with ovarian cancer on same side of family | 0.88 (0.58-1.33) |
a Adjusted for age (continuous), race, facility, and education.
DISCUSSION
Participation in a breast health risk communication and decision support trial varied by women’s demographic characteristics, specifically by age, race/ethnicity, and education. Despite study efforts to improve recruitment through plain language,24,25 which supports readability of the study materials, accessible materials might be necessary, but not sufficient to achieve a representative sample. Hence, the results from our ongoing trial will reflect the underlying population who participated but might not reflect the behavior patterns observed if all women eligible had participated.
Our study population only included women with at least 1.67% 5-year risk of breast cancer, an elevated risk compared with average risk women. While the study population from Haas et al. included < 10% of women at clinically elevated risk,16 our two populations were similar in participant demographics. Despite differences in breast cancer risk, the similar study demographics suggests that the offer of tailored breast cancer risk information is not sufficient to compel broad and representative participation. Further, these observed patterns of research participation mirror participation statistics from other studies aimed to increase women’s attendance at high-risk breast clinics, where attendance remained low (< 15%) even after targeted invitations following screening mammography.26 Similar to our participation factors, attendance rates increase based on women’s demographics (older, white),27 higher breast cancer risk, or a family history of the disease, and in some studies, moderate levels of anxiety.28
Further, women also need to see the topic and clinical services as personally relevant to them. Lived experience29 and perceived personal relevance2 are associated with higher research participation and clinic attendance rates, suggesting that supporting women’s knowledge of their own current breast cancer risk could be motivational in adoption of preventive health strategies. In our study, eligible participants were told their density status and comparative breast cancer risk (i.e., higher than average) when randomized to the intervention, but not told their numeric breast cancer risk as part of the recruitment process. Without this knowledge of personal risk, neither women’s clinical history nor their family history impacted their participation in the study, which suggests that these factors alone are not sufficiently motivational for participation.
Our intention of studying women at elevated risk of breast cancer was to exclude women potentially at risk of hereditary breast and ovarian cancer (HBOC), based on genetic counseling referrals and genetic testing. Women at risk of HBOC experience different clinical management than for women at elevated risk but without a risk of HBOC.33 While we did not observe an association between the number of relatives diagnosed with breast or ovarian cancer with study participation, we did find that participants with other potential indicators of HBOC risk were less likely to participate. Representing only a handful of women in our analysis, these women might have recognized their own personal risk and appropriately did not identify the study as relevant to them. We did not specifically exclude eligible participants based on self-reported family history alone, as additional information through genetic counseling would be needed to assess for HBOC risk. Clear documentation of complete family history is important to ensure women receive appropriate risk management for their family history background.
Study recruitment might have created unforeseen barriers to participation for some women. We utilized the risk factor questionnaire, which women complete at the time of their mammogram, to efficiently identify risk factors calculate 5- and 10-year risk of breast cancer. From this information, we recruited women on average 4.5 months after a normal screening mammogram. However, a delay in initial recruitment contact created a disconnect in timing and potentially reduced any motivation derived from screening mammography. Further, our survey department needed at least four call attempts to reach about 70% of the eligible sample. In both the intervention and usual care groups, women were excluded from the final study population if they completed a telephone baseline survey but did not complete the consent process online. More educated or resourced women may be more likely to participate, given these barriers of timing, telephone recruitment, and motivation. Future work should consider aligning the timing of participation and create a more seamless experience for the participant from recruitment to delivery of the intervention.
Unfortunately, our study continues a history of breast cancer prevention and control research that disproportionally recruits White, educated women.34 Dean et al. emphasizes the need for incorporating social factors like race/ethnicity into clinical cancer care studies, specifically in breast cancer.35 As an example, the Gail Breast Cancer Risk Assessment Tool36 originally underestimated the risk of breast cancer in Black women. With a revised model now validated in Black women, the Breast Cancer Risk Assessment Tool better discriminates breast cancer risk, and the proportion of Black women now considered at elevated breast cancer risk tripled.37 Due to this historical inaccuracy, women of color, particularly Black women, might be less aware of their potential breast cancer risk.35 Given that women of color have denser breast tissue compared with White women,38,39 the relevance of future tools to support breast health requires considering the potentially unique needs of this population and working harder to ensure that they are represented in ongoing research. This general difference in awareness of breast cancer risk aligns with what has been demonstrated to date regarding breast density awareness. Prior surveys have found lower levels of awareness among women of color compared with White women, as well as those with less education and lower income.40-42 Given that our recruitment materials included density-specific information, this information could have more salience among women who had some awareness of the topic.43
Bringing important health information to all women, regardless of demographic factors, is important to consider in scalability within clinical care. Implementation of breast health education tools like ours might require additional supporting activities to actively engage all women. Several methods have been successful in increasing attendance of breast cancer screening, which could be further refined for this context. Methods to evaluate in future research and scalability include community health advisors or peer counselors,44,45 the use of targeted, tailored, and linguistically appropriate materials,46-48 and research and clinical staff who can support the linguistic and cultural needs of the patients.
While our ongoing trial will have several strengths in supporting women’s breast health, our results will remain limited in generalizability. All women in the study were insured and cared for within an integrated care delivery system, providing care from primary care to specialty including genetics. Our study population does not reflect all US healthcare settings, or women uninsured or publicly insured by Medicaid. Further studies should evaluate the use of breast health information, particularly in underserved communities.
In conclusion, the use of plain language and provision of density status and comparative breast cancer risk information were insufficient in engaging a representative sample of women in a breast cancer prevention trial, particularly across race/ethnicity, age, and educational backgrounds. Additional methods of targeting and tailoring, facilitated by clinical and community outreach, are needed to equitably scale breast health interventions within clinical care.
Ethics Approval
All study activities were approved by the Georgetown University Institutional Review Board Committee as the Institutional Review Board of record with a partial waiver of informed consent for recruitment activities. The Kaiser Washington State ceded to the Georgetown University Institutional Review Board.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available, as they are still in process for analysis for our main results but are available from the corresponding author on reasonable request.
Acknowledgments
We thank the ENGAGED study participants and Kaiser Permanente clinical partners in the execution of this study.
Footnotes
Disclosure Statement: The authors have no conflicts of interest to disclose.
Funding: This study is supported by the National Cancer Institute under R01CA190221, R50CA211115, and P30CA05100 and the Agency for Healthcare Research and Quality under K12HS022982. Collection of breast cancer risk information is supported by the National Cancer Institute-funded BCSC (P01CA154292, HHSN261201100031C, and U54CA163303). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors’ Contributions: Substantial contributions to the conception or design of the work were made by KJW, KAL, MDS, and SCO; the acquisition, analysis, or interpretation of data for the work was done by KJW, EAB, SK, KAL, HG, MDS, and SCO; drafting the work or revising it critically for important intellectual content was done by KJW, EAB, SK, KAL, HG, MDS, and SCO; and final approval of the version to be published was provided by KJW, EAB, SK, KAL, HG, MDS, and SCO.
References
- 1.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst 2010;102(16):1224-37. DOI: 10.1093/jnci/djq239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd NF, Martin LJ, Rommens JM, et al. . Mammographic density: A heritable risk factor for breast cancer. Methods Mol Biol 2009;472:343-60. DOI: 10.1186/s12889-019-7566-7 [DOI] [PubMed] [Google Scholar]
- 3.Byrne C, Schairer C, Wolfe J, et al. . Mammographic features and breast cancer risk: Effects with time, age and menopause status. J Natl Cancer Inst 1995;87(21):1622-9. DOI: 10.1093/jnci/87.21.1622 [DOI] [PubMed] [Google Scholar]
- 4.Pankow JS, Vachon CM, Kuni CC, et al. . Genetic analysis of mammographic breast density in adult women: Evidence of a gene effect. J Natl Cancer Inst 1997;89(8):549-56. DOI: 10.1093/jnci/89.8.549 [DOI] [PubMed] [Google Scholar]
- 5.Vachon CM, van Gils CH, Sellers TA, et al. . Mammographic density, breast cancer risk and risk prediction. Breast Cancer Res 2007;9(6):217. DOI: 10.1186/bcr1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capello NM, Richetelli D, Lee CI. The impact of breast density reporting laws on women’s awareness of density-associated risks and conversations regarding supplemental screening with providers. J Am Coll Radiol. 2019;16(2):139-46. DOI: 10.1016/j.jacr.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 7.Are You Dense Advocacy, Inc. State Density Reporting Efforts 2008 [cited 2020 Sept 8]. Available from: https://www.areyoudenseadvocacy.org/dense.
- 8.U.S. Food and Drug Administration. Mammography Quality Standards Act 2018 [cited 2020 Nov 13]. Available from: https://www.fda.gov/media/74251/download.
- 9.Busch SH, Hoag JR, Aminawung JA, et al. . Association of state dense breast notification laws with supplemental testing and cancer detection after screening mammography. Am J Public Health 2019;109(5):762-7. DOI: 10.2105/AJPH.2019.304967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chau SL, Alabaster A, Luikart K, Brenman LM, Habel LA. The effect of California’s breast density notification legislation on breast cancer screening. J Prim Care Community Health 2017;8(2):55- 62. DOI: 10.1177/2150131916674889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas JS. Breast density legislation and the promise not attained. J Gen Intern Med 2019;34(2):167- 8. DOI: 10.1007/s11606-018-4754-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning M, Albrecht TL, O’Neill S, Purrington K. Between-race differences in supplemental breast cancer screening before and after Breast Density Notification Law. J Am Coll Radiol 2019;16(6):797- 803. DOI: 10.1016/j.jacr.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayak L, Miyake KK, Leung JW, Price ER, Liu YI, Joe BN, et al. . Impact of breast density legislation on breast cancer risk assessment and supplemental screening: A survey of 110 radiology facilities. Breast J 2016;22(5):493-500. DOI: 10.1111/tbj.12624 [DOI] [PubMed] [Google Scholar]
- 14.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med 2008;148(5):337-47. DOI: 10.7326/0003-4819-148-5-200803040-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tice JA, Bissell MCS, Miglioretti DL, et al. . Validation of the breast cancer surveillance consortium model of breast cancer risk. Breast Cancer Res Treat 2019;175(2):519-23. DOI: 10.1007/s10549-019-05167-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas JS, Barlow WE, Schapira MM, et al. . Primary care providers’ beliefs and recommendations and use of screening mammography by their patients. J Gen Intern Med 2017;32(4):449-57. DOI: 10.1007/s11606-016-3973-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: Risk assessment to identify women at high risk of breast cancer. J Clin Oncol 2015;33(28):3137-43. DOI: 10.1200/jco.2015.60.8869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consortium. Breast Cancer Surveillance Consortium Risk Calculator V2 2015. [cited 2020 Sept 8]. Available from: https://tools.bcsc-scc.org/bc5yearrisk/calculator.htm. [Google Scholar]
- 19.American College of Radiology. ACR BI-RADS® Atlas [cited 2020 Sept 8]. Available from: https://www.acr.org/Quality-Safety/Resources/BIRADS.
- 20.Knerr S, Wernli KJ, Leppig K, Ehrlich K, Graham AL, Farrell D, et al. . A web-based personalized risk communication and decision-making tool for women with dense breasts: Design and methods of a randomized controlled trial within an integrated health care system. Contemp Clin Trials 2017;56:25-33. DOI: 10.1016/j.cct.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. National Library of Medicine . ENGAGED 2 Study: Experiences With Mammography Screening and Breast Density 2. 2017 [cited 2020 Sept 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT03029286. [Google Scholar]
- 22.KPWHRI. Program for Readability in Science & Medicine (PRISM): Kaiser Permanente Washington Health Research Institute; 2018. [cited 2020 Sept 8]. Available from: https://www.kpwashingtonresearch.org/about-us/capabilities/research-communications/prism/. [Google Scholar]
- 23.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 24.Ridpath JR, Larson EB, Greene SM. Can integrating health literacy into the patient-centered medical home help us weather the perfect storm? J Gen Intern Med 2012;27(5):588-94. DOI: 10.1007/s11606-011-1964-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridpath JR, Wiese CJ, Greene SM. Looking at research consent forms through a participant-centered lens: The PRISM readability toolkit. Am J Health Promot 2009;23(6):371-5. DOI: 10.4278/ajhp.080613-cit-94 [DOI] [PubMed] [Google Scholar]
- 26.Vaidya AM, Chetlen AL, Schetter SE. Does a high-risk recommendation in mammography reports increase attendance at a breast cancer risk assessment clinic? J Am Coll Radiol 2015;12(9):923-9. DOI: 10.1016/j.jacr.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 27.McCaskill-Stevens W, Wilson JW, Cook ED, et al. . National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene trial: Advancing the science of recruitment and breast cancer risk assessment in minority communities. Clin Trials 2013;10(2):280-91. DOI: 10.1177/1740774512470315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ormseth SR, Wellisch DK, Arechiga AE, Draper TL. Predicting reattendance at a high-risk breast cancer clinic. Palliat Support Care 2015;13(5):1441-8. DOI: 10.1017/s1478951515000164 [DOI] [PubMed] [Google Scholar]
- 29.Holmberg C, Waters EA, Whitehouse K, Daly M, McCaskill-Stevens W. My lived experiences are more important than your probabilities: The role of individualized risk estimates for decision making about participation in the Study of Tamoxifen and Raloxifene (STAR). Med Decis Making 2015;35(8):1010-22. DOI: 10.1177/0272989x15594382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinicrope PS, Patten CA, Bonnema SM, et al. . Healthy women’s motivators and barriers to participation in a breast cancer cohort study: A qualitative study. Ann Epidemiol 2009;19(7):484-93. DOI: 10.1016/j.annepidem.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linden HM, Reisch LM, Hart A, Jr., et al. . Attitudes toward participation in breast cancer randomized clinical trials in the African American community: A focus group study. Cancer Nurs 2007;30(4):261-9. DOI: 10.1097/01.ncc.0000281732.02738.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allicock M, Graves N, Gray K, Troester MA. African American women’s perspectives on breast cancer: Implications for communicating risk of basal-like breast cancer. J Health Care Poor Underserved 2013;24(2):753-67. DOI: 10.1353/hpu.2013.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Preventive Services Task Force . BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing 2013. [cited 2020 Sept 8]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. [Google Scholar]
- 34.Vernon SW, Yeomans AC, Frankowski R, Weber D, Vogel VG. Behavioral and social factors that predict participation in the Breast Cancer Prevention Trial. Ann N Y Acad Sci 1995;768:300. DOI: 10.1111/j.1749-6632.1995.tb12146.x [DOI] [PubMed] [Google Scholar]
- 35.Dean LT, Gehlert S, Neuhouser ML, et al. . Social factors matter in cancer risk and survivorship. Cancer Causes Control 2018;29(7):611-8. DOI: 10.1007/s10552-018-1043-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gail MH, Brinton LA, Byar DP, et al. . Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81(24):1879-86. DOI: 10.1093/jnci/81.24.1879 [DOI] [PubMed] [Google Scholar]
- 37.Gail MH, Costantino JP, Pee D, et al. . Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst 2007;99(23):1782-92. DOI: 10.1093/jnci/djm223 [DOI] [PubMed] [Google Scholar]
- 38.McCarthy AM, Keller BM, Pantalone LM, et al. . Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108(10). DOI: 10.1093/jnci/djw104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oppong BA, Dash C, O’Neill S, et al. . Breast density in multiethnic women presenting for screening mammography. Breast J 2018;24(3):334-8.DOI: 10.1111/tbj.12941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Neill SC, Leventhal KG, Scarles M, et al. . Mammographic breast density as a risk factor for breast cancer: Awareness in a recently screened clinical sample. Womens Health Issues 2014;24(3):e321-6. DOI: 10.1016/j.whi.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes DJ, Jenkins SM, Hruska CB, Vachon CM, Breitkopf CR. Breast density awareness, knowledge, and attitudes among U.S. women: National survey results across 5 years. J Am Coll Radiol 2019:S1546-440(19)31271-2. DOI: 10.1016/j.jacr.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 42.Santiago-Rivas M, Benjamin S, Andrews JZ, Jandorf L. Breast density awareness and knowledge, and intentions for breast cancer screening in a diverse sample of women age eligible for mammography. J Cancer Educ. 2019;34(1):90-7. DOI: 10.1007/s13187-017-1271-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahorter SS, Knerr S, Bowles EJA, et al. . Prior breast density awareness, knowledge, and communication in a health system-embedded behavioral intervention trial. Cancer 2020: DOI: 10.1002/cncr.32711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riehman KS, Fisher-Borne M, Martinez JM, et al. . A community health advisor program to reduce cancer screening disparities in the Deep South and Appalachia: The American Cancer Society’s CHA Collaborative. Health Promot Pract 2017;18(5):734-40. DOI: 10.1177/1524839917696712 [DOI] [PubMed] [Google Scholar]
- 45.Fowler BA, Rodney M, Roberts S, Broadus L. Collaborative breast health intervention for African American women of lower socioeconomic status. Oncol Nurs Forum 2005;32(6):1207-16. DOI: 10.1188/05.onf.1207-1216 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen-Truong CKY, Pedhiwala N, Nguyen V, et al. . Feasibility of a Multicomponent Breast Health Education Intervention for Vietnamese American Immigrant Women. Oncol Nurs Forum 2017;44(5):615-25. DOI: 10.1188/17.onf.615-625 [DOI] [PubMed] [Google Scholar]
- 47.Lee EE, Brecht ML, Park H, Lee J, Oh KM. Web-based study for improving mammography among Korean American women. J Cancer Educ 2017;32(2):257-63. DOI: 10.1007/s13187-015-0920-2 [DOI] [PubMed] [Google Scholar]
- 48.Wang JH, Sheppard VB, Liang W, Ma GX, Maxwell AE. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials 2014;11(2):167-77. DOI: 10.1177/1740774513518849 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available, as they are still in process for analysis for our main results but are available from the corresponding author on reasonable request.


