Figure 1. SATB2 interacts with the INM protein LEMD2.
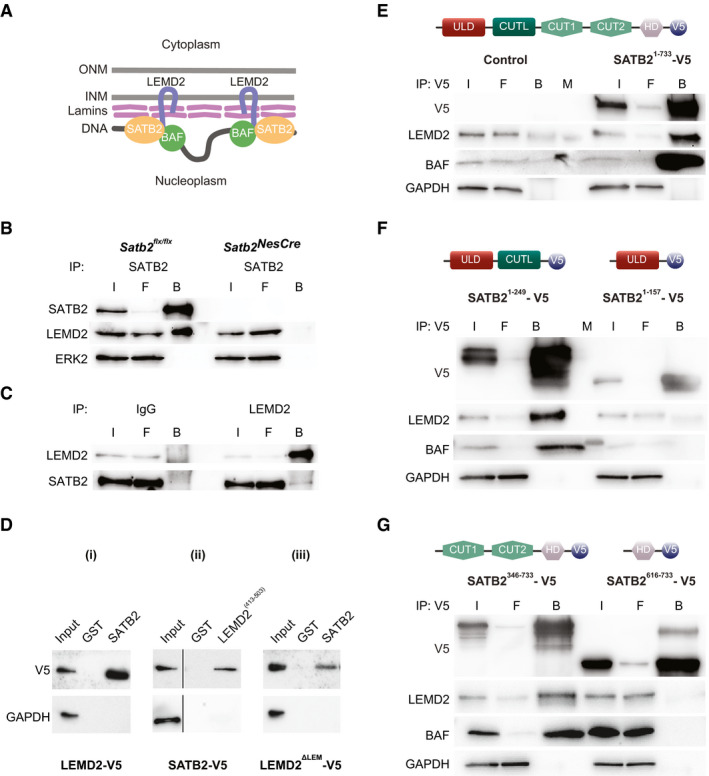
- Model of a hypothetical NL‐chromatin tether containing SATB2. Schematic representation of the nuclear envelope consisting of two lipid bilayers, the inner nuclear membrane (INM) and outer nuclear membrane (ONM), and the lamin polymer underlying the INM (modified after Zuleger et al, 2011). Depicted are the INM protein LEMD2, lamins and BAF, identified as SATB2 interaction partners (Cera et al, 2019).
- Immunoprecipitation of SATB2 from mouse neonatal cortical lysates. LEMD2 was detected by Western blotting in the SATB2 immunoprecipitate from control but not SATB2‐deficient cortical lysate. The equal input of total protein from control and SATB2‐deficient cortical lysates was controlled by ERK2 detection. Representative images of the immunoblots are shown; I (Input), F (Flow‐through), B (Beads).
- Reverse immunoprecipitation with LEMD2 antibody and control IgG antibody, followed by WB detection of LEMD2 and SATB2. Lysates from primary cortical culture lysates were used.
- GST pull‐down assays. Representative images of n = 3 independent experiments are shown. GST pull‐down assay of transiently overexpressed V5‐tagged LEMD2 (i), SATB2 (ii), and LEMD2ΔLEM (iii) from HeLa cell lysates using GST, GST‐SATB2, and GST‐LEMD2(413–503) hybrid proteins.
- Immunoprecipitations using anti‐V5‐tag antibody from lysates of HeLa cells transfected with expression plasmids encoding V5‐tagged full‐length SATB2 (Satb21–733) and EGFP as control. The equal input of total protein was controlled by GAPDH detection. Representative images of the immunoblots are shown; I (Input), F (Flow‐through), B (Beads), M (Molecular weight marker).
- The CUT‐like domain of SATB2 is required for the interaction with LEMD2. Immunoprecipitations using anti‐V5‐tag antibody from lysates of HeLa cells transfected with V5‐tagged Satb21–247 and Satb21–157 deletion mutants. LEMD2 and BAF were detected only in immunoprecipitates from HeLa cells transfected with the deletion mutant containing the CUT‐like domain (Satb21–247). The equal input of total protein was controlled by GAPDH detection. Representative images of the immunoblots are shown; I (Input), F (Flow‐through), B (Beads), M (Molecular weight marker).
- CUT1 and CUT2 domains are required for the interaction with LEMD2. Immunoprecipitations using anti‐V5‐tag antibody from HeLa cells transfected with V5‐tagged Satb2346–733 and Satb2616–733 deletion mutants. LEMD2 and BAF were detected only in immunoprecipitates from HeLa cells expressing the CUT1 and CUT2 containing deletion mutant (Satb2346–733).
Source data are available online for this figure.
