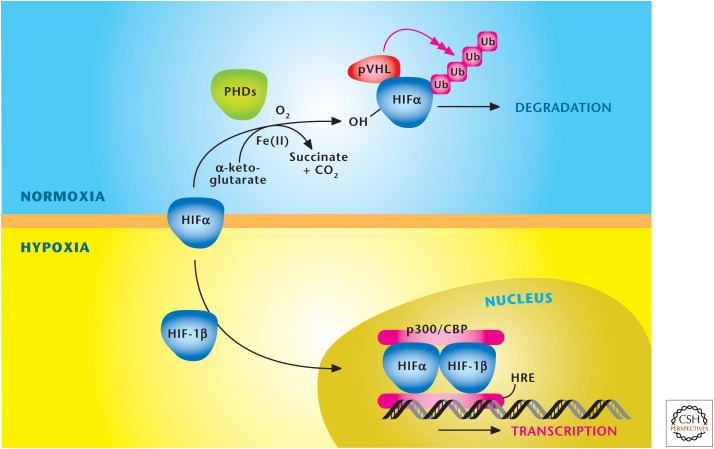
Figure 7.
Oxygen levels control stabilization of HIFs. HIFs are a heterodimer, consisting of a constitutively stable HIF-1β and an oxygen-sensitive HIFα subunit. The HIFα subunit is hydroxylated at proline residues in the ODD by PHDs under normoxia. In addition to oxygen, the PHDs require iron (Fe2+) and α-ketoglutrate as substrates for hydroxylation. The von Hippel–Lindau protein (pVHL) recognizes hydroxylated proline residues and targets the HIFα subunit for rapid degradation by the proteasome. Hypoxia diminishes hydroxylation of HIFα to prevent its degradation and promote dimerization with HIF-1β to bind specific hypoxia response elements (HRE) in the promoter regions of its target genes. (Adapted, with permission, from Balligand et al. 2009, © The American Physiological Society.)