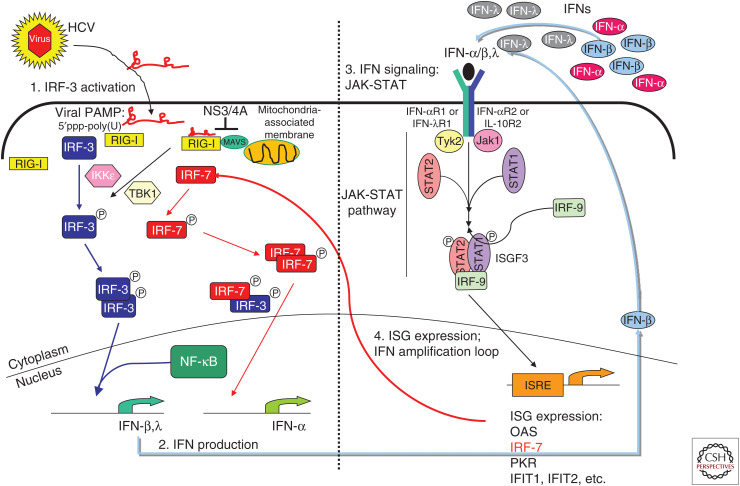
Figure 1.
Sensing and innate immune response to hepatitis C virus (HCV). During acute HCV infection in the hepatocyte, the cytosolic RIG-I-like receptor (RLR), retinoic acid-inducible gene-I (RIG-I), recognizes 5′-triphosphate double-stranded RNA (dsRNA) motifs and/or polyuridine (poly(U)) motifs within HCV RNA. Upon pathogen-associated molecular patterns (PAMPs) binding, RIG-I translocates to the mitochondria-associated membranes (MAMs) where it interacts with the adaptor molecule mitochondrial antiviral-signaling protein (MAVS). Interaction of RIG-I with MAVS at the MAM signaling platform recruits and activates the downstream transcription factors IRF-3 and NF-κB, resulting in the induction of antiviral and immune modulatory type I (interferon [IFN]-α/β) and III (IFN-λ) IFNs. Secreted IFNs bind their cognate receptors IFN-αR and IFN-λR on the cell surface. This autocrine and paracrine IFN signaling engages the JAK/STAT signaling cascade downstream of the IFN receptor and mediates activation and nuclear translocation of the trimeric ISGF3 transcription factor complex, which binds to IFN-stimulated response elements (ISREs) in the promoter regions and induces expression of hundreds of IFN-stimulated genes (ISGs) to establish an antiviral state. The viral NS3/4A protease accumulates in the infected cell and targets and cleaves MAVS, releasing it from the MAM and thus inactivating the RLR pathway. This process allows HCV to evade intracellular innate immunity to mediate persistent viral replication and chronic infection.