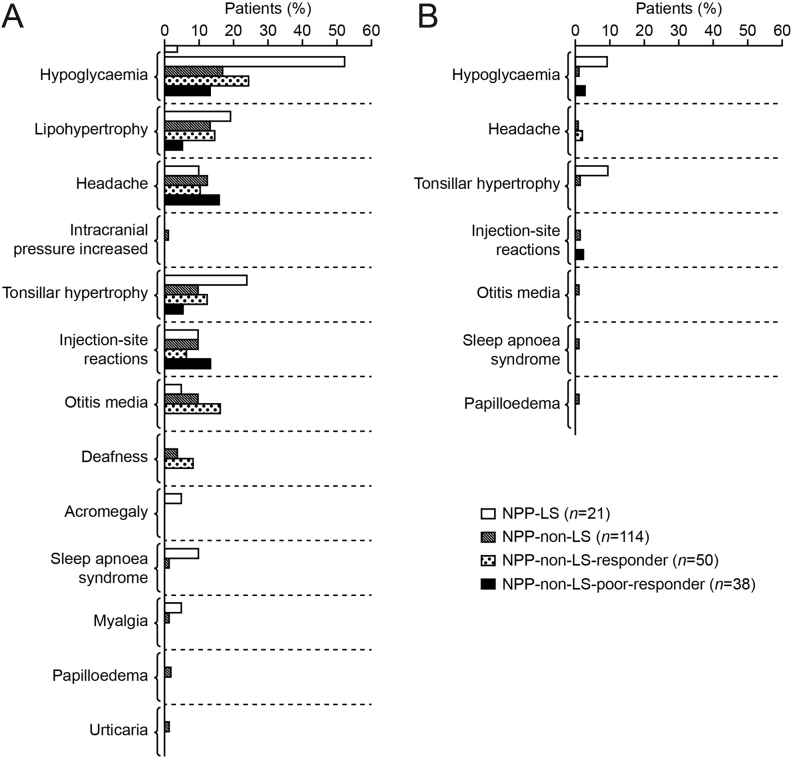
Figure 4.
All targeted TEAEs for treatment-naïve/prepubertal (NPP) patient subgroups (A) total, (B) serious (safety population). All reported targeted TEAEs and serious targeted TEAEs are shown. Targeted TEAEs were: headache, chronic middle ear infection, papilledema, hypoglycaemia, acromegalic facial changes, oedema, gynaecomastia, hearing loss, intracranial hypertension, lipohypertrophy at injection sites, myalgia, sleep apnoea, tonsillar hypertrophy and cardiomegaly. Hearing impairment was coded as deafness. Responders were defined as patients with a change in height SDS in year 1 of ≥0.3. Poor-responders were defined as patients with a change in height SDS in year 1 of <0.3. LS, Laron syndrome; TEAE, treatment-emergent adverse event.

 This work is licensed under a
This work is licensed under a