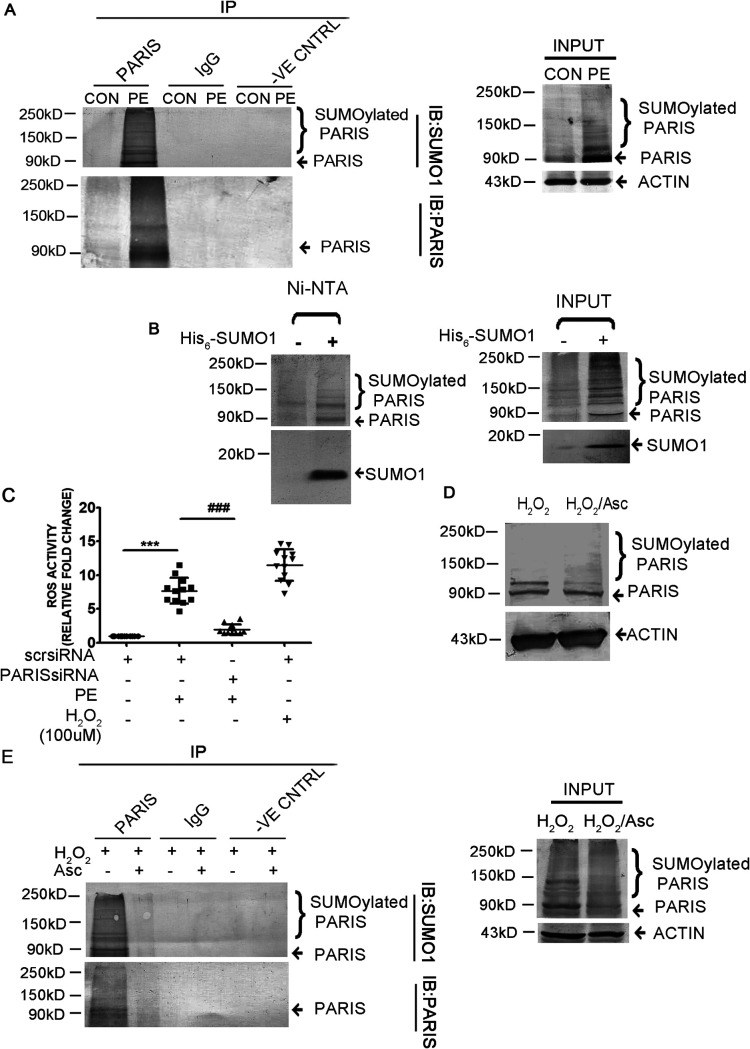
FIG 6.
The function of PARIS is regulated by SUMOylation in hypertrophied cardiomyocytes. (A) Coimmunoprecipitation of control and PE-treated cell lysates extracted under denaturing conditions using anti-PARIS antibody followed by immunoblotting with anti-SUMO1 antibody showing increased SUMOylation of PARIS during hypertrophy. Control beads and IgG served as negative controls. Untreated control and PE-treated cell lysates served as input. The data represent 3 independent experiments. (B) HEK293 cells were transfected with His6-SUMO1. Transfected cells were lysed under denaturing conditions, and the SUMO1-modified proteins were purified by chromatography on Ni-NTA–agarose beads followed by immunoblotting using anti-PARIS antibody, showing slower-migrating bands of SUMOylated PARIS (lane 2). n = 3 for each group from 3 independent experiments. (C) Measurement of ROS content using a DCFDA assay kit in H9C2 cells in different treatment groups showing significantly reduced ROS level in PARIS siRNA treatment; H2O2 was used as a positive control. n = 12 for each group from 4 independent experiments. ***, P < 0.001 compared to controls; ###, P < 0.001 compared to PE treatment. (D) Western blot demonstrating decreased SUMOylation of PARIS in ascorbate treatment compared to H2O2-treated H9C2 cells. Actin was used as the loading control. n = 3 for each group from 3 independent experiments. (E) Coimmunoprecipitation under denaturing conditions using anti-PARIS antibody followed by immunoblotting with anti-SUMO1 antibody in different treatment groups showing reduced SUMOylation of PARIS in cells in which ROS had been scavenged. Control beads and IgG served as negative controls. H2O2- and H2O2-Asc-treated cell lysed under denaturing condition served as input. n = 3 for each group from 3 independent experiments.