Although vitamin D is critical for the function of the intestine, most studies have focused on the duodenum. We show that transgenic expression of the vitamin D receptor (VDR) only in the distal intestine of VDR null mice (KO/TG mice) results in the normalization of serum calcium and rescue of rickets. Although it had been suggested that calcium transport in the distal intestine involves a paracellular process, we found that the 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]-activated genes in the proximal intestine associated with active calcium transport (Trpv6, S100g, and Atp2b1) are also induced by 1,25(OH)2D3 in the distal intestine of KO/TG mice.
KEYWORDS: calcium, manganese, transporters, intestine, vitamin D, human enteroids, mouse, Slc30a10, TRPV6
ABSTRACT
Although vitamin D is critical for the function of the intestine, most studies have focused on the duodenum. We show that transgenic expression of the vitamin D receptor (VDR) only in the distal intestine of VDR null mice (KO/TG mice) results in the normalization of serum calcium and rescue of rickets. Although it had been suggested that calcium transport in the distal intestine involves a paracellular process, we found that the 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]-activated genes in the proximal intestine associated with active calcium transport (Trpv6, S100g, and Atp2b1) are also induced by 1,25(OH)2D3 in the distal intestine of KO/TG mice. In addition, Slc30a10, encoding a manganese efflux transporter, was one of the genes most induced by 1,25(OH)2D3 in both proximal and distal intestine. Both villus and crypt were found to express Vdr and VDR target genes. RNA sequence (RNA-seq) analysis of human enteroids indicated that the effects of 1,25(OH)2D3 observed in mice are conserved in humans. Using Slc30a10−/− mice, a loss of cortical bone and a marked decrease in S100g and Trpv6 in the intestine was observed. Our findings suggest an interrelationship between vitamin D and intestinal Mn efflux and indicate the importance of distal intestinal segments to vitamin D action.
INTRODUCTION
The essential role of vitamin D is to mediate calcium homeostasis through its regulatory effects on intestine, bone, and kidney (1). Vitamin D has also been reported to affect numerous other physiological processes not involved in calcium regulation, including immunomodulatory effects and inhibition of cancer progression (1). The skeletal and extraskeletal effects of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (the biologically active from of vitamin D [calcitriol]) are mediated by the vitamin D receptor (VDR), a nuclear protein which heterodimerizes with the retinoid X receptor (RXR). VDR/RXR interacts with DNA sequences and vitamin D response elements (VDREs) in target genes and leads to activation or repression of transcription. 1,25(OH)2D3 also recruits coregulatory complexes that participate in the regulation of transcription of VDR target genes. VDR-mediated gene transcription is a multistep process requiring the spatial and sequential combination of transcriptional coactivators and is influenced by the particular subcellular environment in a gene-specific manner (2, 3).
A principal target tissue of vitamin D action is the intestine (1, 4, 5). However, little is known about the molecular targets mediating the effects of vitamin D on intestinal biology. The classic role of 1,25(OH)2D3 is the regulation of intestinal calcium absorption, which is crucial for bone health. When the need for calcium increases, for example, during periods of habitual low calcium intake and during growth, vitamin D-mediated intestinal calcium absorption occurs predominantly by an active transcellular process. The most pronounced effects of 1,25(OH)2D3 during the active calcium transport process are increased synthesis of the epithelial calcium channel TRPV6 and the intracellular calcium binding protein calbindin-D9k (4, 5). In addition to the active transcellular pathway, calcium can also be absorbed via a nonsaturable paracellular process (4, 5). Whether 1,25(OH)2D3 regulates calcium absorption by modulating genes associated solely with the facilitated diffusion model or whether 1,25(OH)2D3 affects the paracellular process has been a matter of debate (6–9). Most research on calcium absorption has utilized the proximal small intestine, and early studies reported that 1,25(OH)2D3-regulated active calcium transport was localized only in the duodenum (9). It had been suggested that calcium absorption in the distal intestine reflects vitamin D-independent passive diffusion (10). However, previous studies also noted that 1,25(OH)2D3 and low dietary calcium can regulate active calcium absorption in the distal intestines of rats (11–13). In addition, we recently showed that transgenic expression of VDR only in the distal intestine (distal ileum, cecum, and colon) at levels equivalent to those in wild-type (WT) mice results in normal calcium homeostasis and reverses the VDR-dependent rickets of the VDRnull mice (14). These findings indicate the importance of the distal intestinal segments to vitamin D action.
Although the classical intestinal role of 1,25(OH)2D3 is the regulation of calcium absorption, 1,25(OH)2D3 has been reported to have other important effects on the intestine, including the protection of the integrity of the mucosal barrier, regulation of pathogen invasion, apoptosis, nutrient transport, and cellular differentiation (7, 15–19). However, the molecular responses to 1,25(OH)2D3 resulting in these effects in proximal versus those in distal intestine are poorly understood. In addition, although intestinal epithelial cells at the villi have been identified as targets for 1,25(OH)2D3, little is known about the effect of 1,25(OH)2D3 in crypts; whether 1,25(OH)2D3 responses occur in crypts has been a matter of debate (20–22). Thus, the diversity and complexity of vitamin D signaling in the intestine have not been fully clarified.
In this study, to further understand the role of vitamin D in the intestine, we examined the expression of VDR targets using transgenic mice with expression of VDR restricted to the distal intestine (KO/TG mice) (14) and compared our findings in these transgenic mice to vitamin D targets in both proximal and distal intestines of vitamin D-deficient mice treated with 1,25(OH)2D3. The classic 1,25(OH)2D3-activated genes found in the proximal intestine and associated with active calcium transport (Trpv6, S100g, and Atp2b1) were also induced in the distal intestine. In addition, one of the genes most induced by 1,25(OH)2D3 in the proximal and distal intestine was Slc30a10, a manganese (Mn) efflux transporter which has recently been found to be critical for protection against neurotoxicity and liver injury which occur in the presence of elevated Mn levels (23). Both villus and crypts were found to express high levels of VDR and result in 1,25(OH)2D3-mediated target gene induction. RNA sequence (RNA-seq) analysis of human enteroids confirmed the induction of the same genes in mice and humans (TRPV6, SLC30A10, ATP2B1, and CYP24A1) in both villus and crypts, indicating, for the first time, direct transcriptomic responses to 1,25(OH)2D3 in human enteroids in both crypt and villus-like compartments. Studies in Slc30a10−/− mice showed a loss of cortical bone and a marked decrease in S100g and Trpv6 in the intestine of these mice. Our findings suggest that 1,25(OH)2D3 may have a role not only to maintain calcium homeostasis but also in the cellular homeostasis of other divalent ions. Our findings emphasize the importance of the distal as well as the proximal intestine in order to understand intestinal effects of 1,25(OH)2D3 and show that the effects of 1,25(OH)2D3 involve intestinal epithelial cells in both villus and crypt.
RESULTS
RNA-seq analysis of transcriptomic responses to 1,25(OH)2D3 in the colons of mice with transgenic expression of VDR only in the distal intestine.
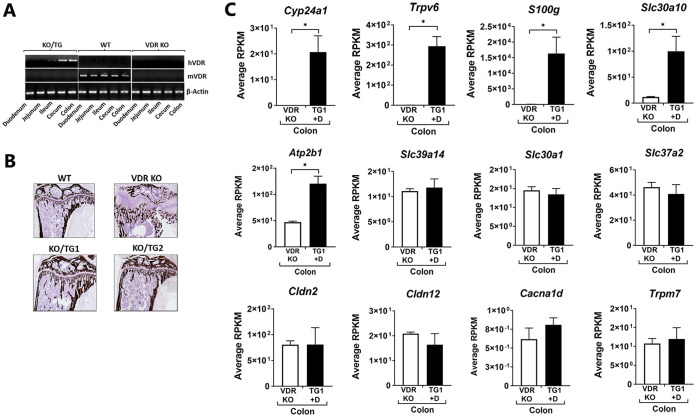
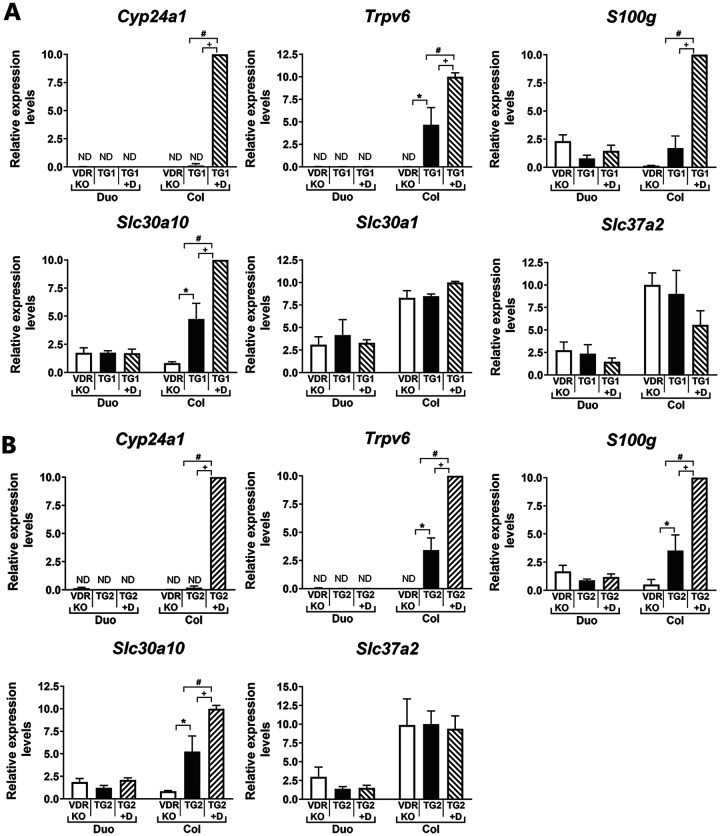
Although most studies have focused on calcium absorption in the proximal intestine, our previous study demonstrated that transgenic expression of VDR only in the distal intestine rescued VDR-dependent rickets observed in the VDR knockout (KO) mouse (14). To understand mechanisms by which 1,25(OH)2D3 acts to regulate intestinal biology not only in the proximal but also in the distal intestine, transcriptomic profiling was conducted to examine the expression of genes induced by 1,25(OH)2D3 in the distal intestine of KO/TG mice (KO/TG line 1, noted as TG1), which express VDR exclusively in the distal intestine (distal ileum, cecum, and colon) (14) (Fig. 1A and B). We focused on the colon as a possible unappreciated target for regulation of calcium homeostasis. As noted in Fig. 1C the classic 1,25(OH)2D3-activated genes in the proximal intestine were also the genes most markedly induced by 1,25(OH)2D3 in the colon of the TG mice (Cyp24a1, Trpv6, and S100g). Induction of S100g andTrpv6 as well as Atp2b, genes involved in active calcium transport (1), suggests that active calcium transport in the distal intestine is involved in the normalization of mineral homeostasis in these mice. Genes associated with passive calcium transport, Cldn2, Cldn12 or Cacna1d (Cav1.3 gene), and Trpm7, which have been suggested as channels other than Trpv6 for the regulation of intestinal calcium absorption (6, 24, 25), were not significantly regulated by 1,25(OH)2D3 in the colon. In addition to the classic 1,25(OH)2D3-activated genes, Slc30a10, the gene for a manganese efflux transporter located in the apical/luminal domain, was also one of the genes most induced by 1,25(OH)2D3 and was the principal Slc transporter gene affected by 1,25(OH)2D3. Other Slc transporter genes, including Slc30a1 (a Zn transporter) as well as Slc30a4 and Slc30a5 (also Zn transporters [not shown]), Slc39a14 (a Mn transporter which mediates Mn reuptake from the basolateral membrane), and Slc37a2 (a phosphate-linked glucose 6 phosphate antiporter), were not significantly affected by 1,25(OH)2D3. Real-time PCR (RT-PCR) analysis of intestinal tissues from the same transgenic mouse line (KO/TG1) (Fig. 2A) as well as from a second independent KO/TG line (KO/TG2, noted as TG2) (Fig. 2B) validated the RNA-seq analyses, indicating that the genes most responsive to 1,25(OH)2D3 in the distal intestine of KO/TG mice include Cyp24a1, Trpv6, S100g, and Slc30a10. Other Slc transporter genes, Slc30a1 and Slc37a2, were unaffected by 1,25(OH)2D3 (Fig. 2). Due to the expression of VDR only in the distal intestine, the duodenum of KO/TG mice was unresponsive to 1,25(OH)2D3 (Fig. 2, Duo).
FIG 1.
Gene expression measured by RNA-seq in the colon of KO/TG1 mice treated with 1,25(OH)2D3. (A) KO/TG mice express VDR only in the distal intestine. Expression of hVDR is restricted to the distal intestines of KO/TG mice. Mouse (m) VDR is absent in KO/TG mice. (B) Rescue of VDR-dependent rickets in the KO/TG mice (Von Kossa staining [14]). (C) Gene expression in the colon of KO/TG mice treated with 1,25(OH)2D3 (TG1 +D) compared to that in VDR KOs. Gene expression, measured by RNA-seq analysis, is shown as the mean of reads per kilobase per million (RPKM) ± SEM. n = 4 per group *, P < 0.05.
FIG 2.
Response to 1,25(OH)2D3 treatment in KO/TG mouse intestine as measured by RT-qPCR. Gene expression in the duodenum (Duo) and colon (Col) of KO/TG1 mice (TG1) (A) or KO/TG2 mice (mice from a second transgenic line [TG2]) (B) treated with vehicle or 1,25(OH)2D3 (1 ng/g body weight at 48, 24, and 6 h before termination; TG1+ D or TG2 + D). Results are compared to those from VDR KO mice. n = 6 per experimental group. Gene expression was normalized to Gapdh and expressed as means ± SEMs. +, P < 0.05 compared to vehicle (TG1 or TG2); #, P < 0.05 between 1,25(OH)2D3 treated (TG1 or TG2 +D) and VDR KO; *, P < 0.05 between vehicle (TG1 or TG2) and VDR KO. The duodenum was included as a control. Note the lack of responsiveness of the duodenum to 1,25(OH)2D3 treatment due to the absence of VDR in the proximal intestine of these transgenic mice. ND, not detected.
Regulation of vitamin D target genes in the intestine.
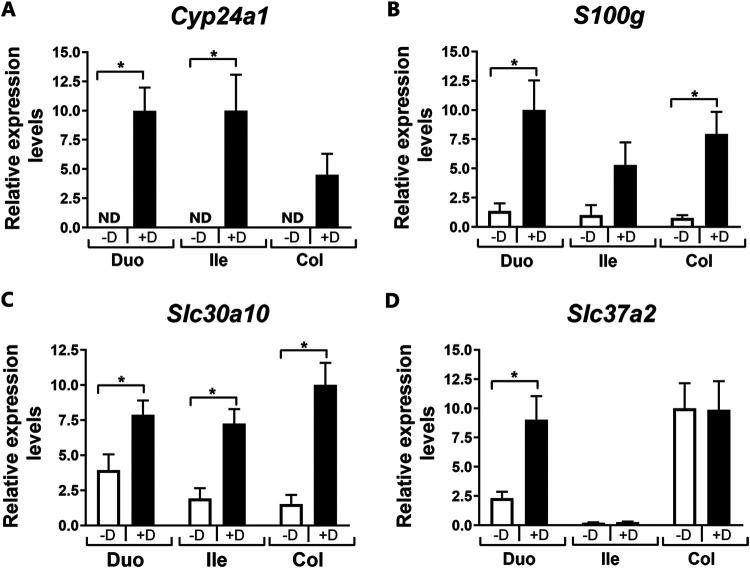
In addition to the induction of Slc30a10 in the distal intestine of transgenic mice, Slc30a10 was found to be regulated by 1,25(OH)2D3 in different intestinal segments (duodenum, ileum, and colon) of vitamin D-deficient mice similarly as other vitamin D target genes (Cyp24a1 and S100g). Slc37a2, although not regulated by 1,25(OH)2D3 in the distal intestine, was induced by 1,25(OH)2D3 in the duodenum (Fig. 3). In addition, in the duodenum expression of Slc30a4, Slc30a5, Slc39a14, Trmp7, Cldn12, and Cacna1d, similar to findings in the colon (Fig. 1), was unaffected by 1,25(OH)2D3 treatment of vitamin D-deficient mice (n = 4; vehicle versus 1,25(OH)2D3 treated, P > 0.5). Cldn2, however, was significantly induced by 1,25(OH)2D3 in the duodenum of these mice (3.8-fold, n = 4; vehicle versus 1,25(OH)2D3 treated, P < 0.05).
FIG 3.
Gene expression in intestinal segments in response to repeated administration of 1,25(OH)2D3 to vitamin D-deficient mice. Twelve- to 14-week-old vitamin D-deficient mice were injected with 1,25(OH)2D3 (+D) or vehicle (−D) three times over 48 h (at 48, 24, and 6 h prior to termination; i.p., 1 ng/g body weight per injection). Data were normalized to Gapdh and expressed as means ± SEMs. n = 6 per experimental group. *, P < 0.05 versus vehicle-treated group; Duo, duodenum; Ile, ileum; Col, colon; ND, not detected.
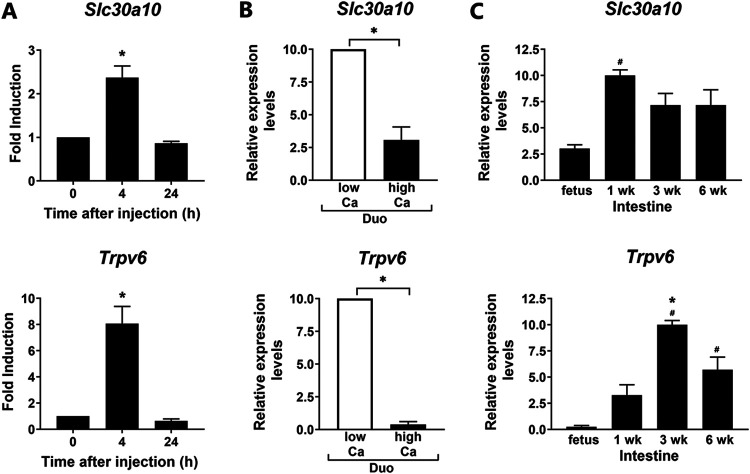
To gain insight into a possible role of vitamin D in the regulation of Mn transport, further studies were conducted comparing the regulation of Slc30a10 in the intestine to the regulation of known vitamin D target genes. As shown in Fig. 4A, Slc30a10 and Trpv6 showed similar time courses of induction by 1,25(OH)2D3. Gene expression for these transporters was significantly induced in the duodenum at 4 h after injection and then decreased to control levels at 24 h after 1,25(OH)2D3 administration. Similar findings were observed for Slc30a10 and Trpv6 in colon (data not shown). These findings suggest a rapid response of Trpv6 and Slc30a10 to 1,25(OH)2D3 for intestinal calcium or Mn transport.
FIG 4.
Regulation of intestinal 1,25(OH)2D3 target gene expression: time course of induction by 1,25(OH)2D3, effects of dietary calcium and developmental changes. (A) RT-qPCR analysis of Slc30a10 and Trpv6 expression in the duodenum at 0, 4, and 24 h after a single injection of 1,25(OH)2D3 (10 ng/g body weight) to vitamin D-deficient mice. n = 5 per time point. *, P < 0.05 compared to zero time point. (B) RT-qPCR analysis of Slc30a10 and Trpv6 expression in duodenum of mice fed a low-calcium (0.02%) or high-calcium (1%) diet for 4 weeks. n = 7 per group. *, P < 0.05. (C) Developmental changes in gene expression in mice raised under standard conditions and killed at 18 days gestation and at 1, 3, and 6 weeks of age. Intestine was harvested (for fetus, n = 5, pooled intestinal samples of 3 or 4 individual intestines; n = 5 or 6 for 1-, 3-, and 6-week-old mice). Since the small intestine and not individual segments were harvested and analyzed in the fetus, for comparison, small intestine was also analyzed at different postnatal stages. Data are expressed as means ± SEMs. Data were normalized using Gapdh or 18S rRNA. #, P < 0.05 versus fetus; *, P < 0.05 versus 1-week-old rat; Duo, duodenum.
In addition to regulation by 1,25(OH)2D3, effects of dietary calcium on the expression of these genes was also examined. Four-week-old mice were fed either a high-calcium (1%) or low-calcium diet (0.02%) diet for 4 weeks. As shown in Fig. 4B, under conditions of low dietary calcium [which results in increased circulating 1,25(OH)2D3 levels] (1) an induction in both Slc30a10 and Trpv6 was observed in the duodenum. A similar induction of these genes in response to low dietary calcium was also observed in the colon (not shown). S100g was also significantly increased in duodenum and colon in response to low dietary calcium (data not shown). These findings suggest coregulation of these manganese and calcium transporters under conditions of dietary calcium alteration.
In additional studies, developmental changes in the expression of Slc30a10 and Trpv6 were compared. Trpv6 was not expressed significantly in the intestine until after birth and was induced at 3 weeks of age, the time of weaning in mice, which coincides with the increase in active intestinal calcium transport (Fig. 4C) (26). Unlike Trpv6, Slc30a10 was detected in the fetal intestine. The most marked induction in the expression of Slc30a10 in the intestinewas observed betweenfetal samples and at 1 week postpartum. Thus, developmental changes in intestinal Slc30a10 and Trpv6 do not coincide, suggesting that different factors modulate the developmental expression of these transporters.
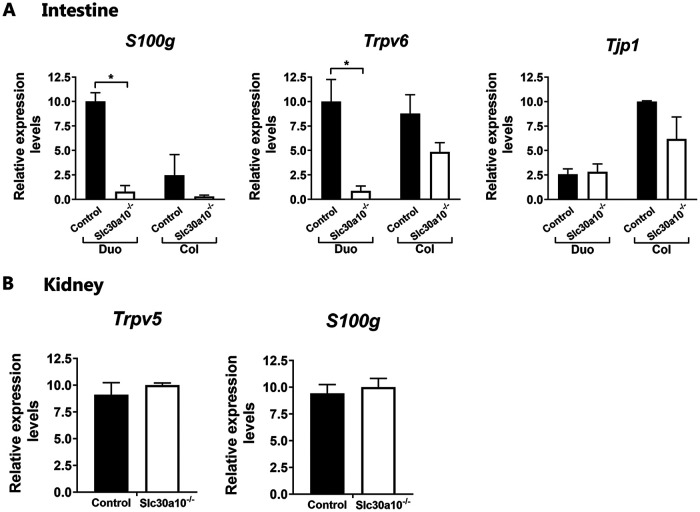
To further understand a possible interrelationship between vitamin D and manganese homeostasis, vitamin D target genes associated with 1,25(OH)2D3-mediated calcium transport were examined in the intestine of the Slc30a10−/− mice, which have manganese levels in blood, brain, and liver 20- to 60-fold higher than do controls (27). Expression of S100g and Trpv6 was markedly decreased in the duodenum of the Slc30a10−/− mice (>10-fold) (Fig. 5A) but not significantly changed in the colon. Also, no change was observed in the duodenum and colon in the expression of Tjp1 [ZO-1 gene; previous reports suggested regulation by 1,25(OH)2D3 of intestinal tight junction protein ZO-1] (28). To determine the specificity for vitamin D-regulated calcium transporters in the intestine, Trpv5 and S100g were examined in the kidneys of Slc30a10−/− mice. Expression of S100g and Trpv5 was unaffected in the kidney (Fig. 5B).
FIG 5.
1,25(OH)2D3 target gene expression in the intestine (A) and kidney (B) of Slc30a10−/− and control mice. Data are expressed as means ± SEMs and were normalized using Gapdh. n = 4 to 6 in each group. *, P < 0.05; Duo, duodenum; Col, colon.
Micro-computed tomography (μCT) analysis of the tibiae was performed to assess trabecular and cortical parameters in control and Slc30a10−/− mice. Trabecular bone mass was slightly, although not significantly, reduced in male Slc30a10−/− mice versus that in control mice, whereas no differences in trabecular bone mass were observed in female mice (Table 1). However, a strong cortical bone phenotype was observed in both male and female mice. Tibias of Slc30a10−/− mice were smaller in diameter than tibias of control mice as evidenced by their reduced cross-sectional tissue area. In addition, cortical thickness as well as cortical porosity were significantly reduced in Slc30a10−/− mice at middiaphysis (Table 1). Serum calcium levels were unchanged between Slc30a10−/− and control mice (12.5 ± 1.3 versus 12.3 ± 0.4 ng/ml, respectively; P > 0.5; n = 5) Thus, in addition to the reduction in Trpv6 and S100g, there was a significant bone phenotype in these mice.
TABLE 1.
Trabecular and cortical bone parameters of control and Slc30a10 KO mice
| Parametera | Value inb: |
|||
|---|---|---|---|---|
| Male mice |
Female mice |
|||
| Control | Slc30a10 KO | Control | Slc30a10 KO | |
| Trabecular bone | ||||
| n | 8 | 5 | 10 | 9 |
| BV/TVc (%) | 6.55 ± 1.60 | 4.44 ± 1.29 | 5.41 ± 2.33 | 4.72 ± 1.20 |
| Trabecular no. (per mm) | 2.07 ± 0.39 | 1.48 ± 0.36d | 1.62 ± 0.57 | 1.54 ± 0.35 |
| Trabecular thickness (mm) | 0.031 ± 0.002 | 0.030 ± 0.002 | 0.033 ± 0.002 | 0.031 ± 0.001d |
| Cortical bone | ||||
| n | 8 | 4 | 10 | 8 |
| Cross-sectional tissue area (mm2) | 2.11 ± 0.30 | 1.35 ± 0.39d | 1.92 ± 0.27 | 1.33 ± 0.16d |
| Medullary area (mm2) | 1.44 ± 0.23 | 1.00 ± 0.34d | 1.29 ± 0.23 | 1.03 ± 0.13d |
| Cortical thickness (mm) | 0.124 ± 0.012 | 0.092 ± 0.023d | 0.140 ± 0.011 | 0.086 ± 0.010d |
| Porosity (%) | 12.07 ± 2.68 | 6.12 ± 1.12d | 9.22 ± 2.07 | 6.24 ± 1.55d |
Trabecular bone parameters and cortical bone parameters as measured by μCT.
Data are presented as means ± SEMs (unpaired, two-tailed t test KO versus WT for each sex).
BV/TV, bone volume/tissue volume.
P < 0.05.
VDR and vitamin D target genes in mouse crypts and villi and in differentiated and undifferentiated human enteroids.
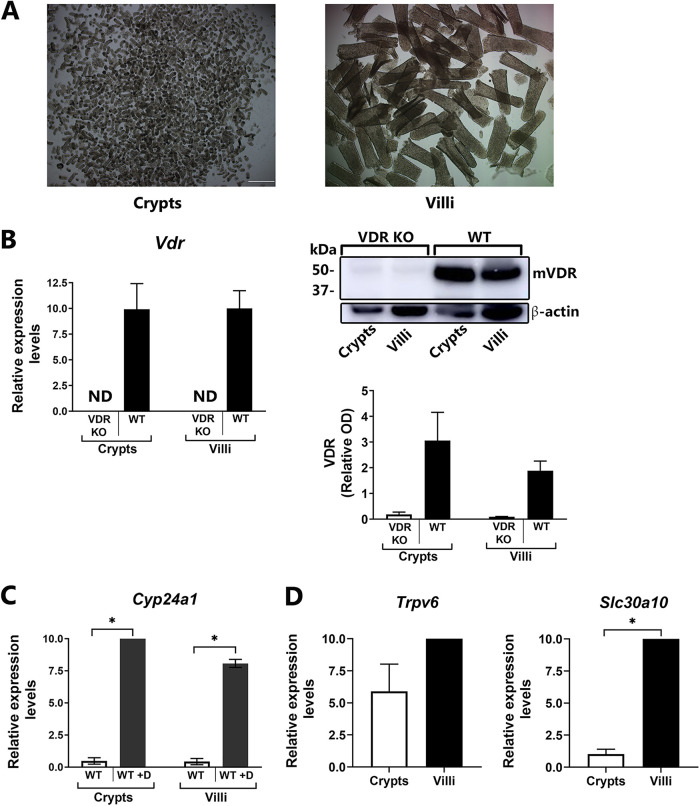
To provide insight into the mechanisms involved in the regulation of vitamin D target genes in the intestine, including the ion transporters, we examined both mouse villi and crypts. Additionally, human enteroid cultures were used to examine both undifferentiated (crypt-like) and differentiated (villus-like) responses to 1,25(OH)2D3 and to determine whether the effects of 1,25(OH)2D3 in the intestine observed in mice are conserved in humans. Although the importance of vitamin D-regulated proteins in intestinal villi has been reported, little is known about effects of 1,25(OH)2D3 in crypts, and few findings related to human intestinal VDR targets have been reported. Crypts and villi were isolated from mouse duodenum using the EDTA chelation method (Fig. 6A). Expression of Vdr as well as VDR protein in mouse villus and crypt was equivalent (Fig. 6B). Both villus and crypt respond to 1,25(OH)2D3 by inducing expression of Cyp24a1 (Fig. 6C). Slc30a10 and Trpv6, although more pronounced in villi, were also expressed in crypts of WT mice (Fig. 6D).
FIG 6.
VDR and 1,25(OH)2D3 target genes in mouse epithelial cells in villi and crypts. (A) Intestinal epithelial cells were isolated from crypts (left) and villi (right) of 3-month-old mouse duodenum. (B) Vdr gene expression (left) and VDR protein (Western blot analysis; right) in mouse duodenal crypts and villi from 3-month-old WT mice compared to that in VDRKO mice. For Western blot analysis, the relative optical density (OD) obtained using the VDR antibody was divided by the OD obtained after β-actin staining to produce VDR-relative OD. n = 3 or 4. (C and D) RT-qPCR analysis of 1,25(OH)2D3 target genes in mouse duodenal villi and crypts. (C) Cyp24a1 expression was examined in 3-month-old mice injected i.p. with vehicle or 1,25(OH)2D3 (1 ng/g body weight; injected at 48, 24, and 6 h prior to euthanasia). *, P < 0.05. (D) Trpv6 and Slc30a10 gene expression in intestinal epithelial cells from crypts and villi of 3-month-old WT mice. RT-qPCR results were normalized to Gapdh or 18S rRNA. n = 3 or 4. ND, not detected.
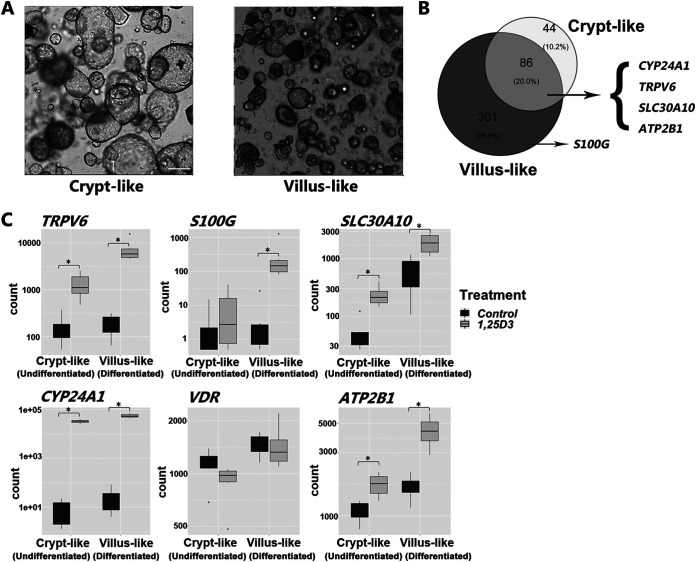
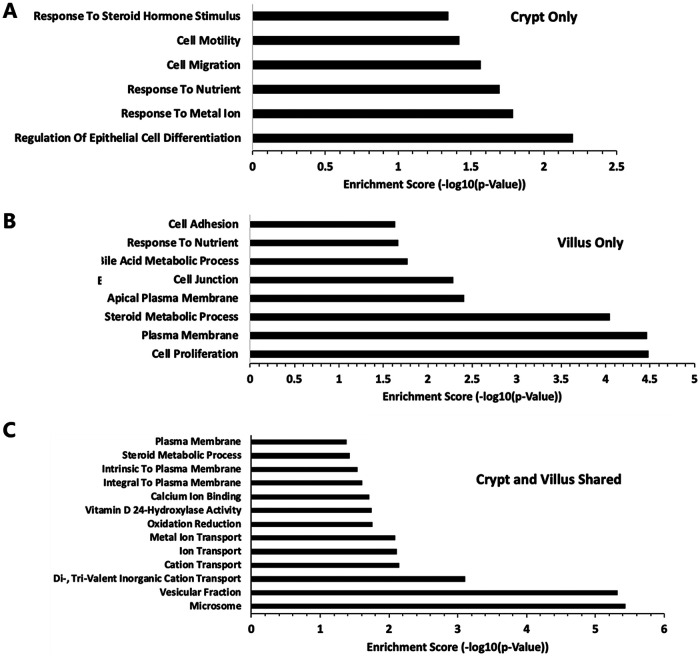
RNA-seq analysis of human duodenal enteroids (Fig. 7) showed that intestinal vitamin D target genes are conserved in humans. Similar to the findings in mice, expression of VDR under basal conditions was equivalent in villus and crypt compartments. The expression of VDR was not significantly affected by 1,25(OH)2D3. However, TRPV6, SLC30A10, CYP24A1, and ATP2B1 were significantly upregulated upon 1,25(OH)2D3 treatment in both villus and crypt (Fig. 7C). S100G was significantly induced by 1,25(OH)2D3 only in villus-like cultures. A similar regulation of vitamin D target genes was observed in crypt-like and villus-like human enteroids derived from the colon (data not shown). Expression of SLC transporter genes in addition to SLC30A10 in differentiated and undifferentiated human duodenal enteroids treated with 1,25(OH)2D3 or vehicle is shown in Table 2. SLC30A4 and SLC30A5 were not significantly affected by 1,25(OH)2D3. SLC37A2 was induced in villus but not in crypts. The intestinal phosphate transporter, encoded by SLC34A2, was upregulated by 1,25(OH)2D3 in both villus and crypt. Expression of TRPM7, a non-SLC gene transporter, was unaffected by 1,25(OH)2D3 treatment. Gene ontology (GO) analysis was performed to identify biological processes, cellular components, and molecular function terms that are enriched in human duodenal enteroids treated with 1,25(OH)2D3. The enrichment scores (−log10 [P value]) of the terms were plotted to indicate the significance of the enrichment of each function. Regulation of epithelial cell differentiation represented a major category for enrichment of genes by 1,25(OH)2D3 in crypts (Fig. 8). Sonic hedgehog (SHH), which controls the proliferation of stem cells, was a 1,25(OH)2D3 target gene in this ontology group and is expressed in response to nutrients, cell migration, and cell motility, indicating a role for 1,25(OH)2D3 in the function of stem cells. Pathway analysis indicated enrichment of genes by 1,25(OH)2D3 involved in ion transport, oxidation reduction, vitamin D 24-hydroxylase activity, and steroid metabolic processes as well as in calcium ion binding, and microsomes and vesicular fraction were categories shared in both villus and crypt (Fig. 8). 1,25(OH)2D3 target gene members of the gene ontology group identifying microsomes and vesicular fraction included CYP3A4, which is involved in vitamin D metabolism, and CYP2C19 and CYP2C18, which are involved in drug metabolism.
FIG 7.
Gene expression measured by RNA-seq in crypt-like and villus-like human duodenal enteroids. (A) Bright-field images of crypt-like (undifferentiated) and villus-like (differentiated) duodenal human enteroids. (B) Venn diagram indicating genes significantly upregulated at least 1.5-fold and with a false-discovery rate [FDR] of <0.05 upon 1,25(OH)2D3 treatment (100 nM for 24 h) in crypt-like (undifferentiated) and villus-like (differentiated) human enteroids and overlapping genes. (C) Expression counts of SLC30A10, VDR, and classic 1,25(OH)2D3 target genes in control and 1,25(OH)2D3-treated villus and crypt-like human enteroids. Data are from enteroids from 6 patients (3 female and 3 male).
TABLE 2.
Gene expression of transporters in crypt-like and villus-like human enteroids derived from duodenum treated with 1,25(OH)2D3 or vehicle
| Gene | Crypt-like enteroid |
Villus-like enteroid |
Description | ||
|---|---|---|---|---|---|
| Fold change | P value | Fold change | P value | ||
| SLC30A10 | 5.38 | 2.05E−19a | 3.20 | 2.99E−03a | Mn efflux transporter |
| SLC30A4 | 0.82 | 5.51E−01 | 0.93 | 8.44E−01 | Zn transporter |
| SLC30A5 | 1.02 | 9.56E−01 | 1.05 | 7.89E−01 | Zn transporter |
| SLC34A2 | 6.94 | 5.17E−22a | 3.02 | 3.00E−03a | Phosphate transporter |
| SLC37A2 | 1.06 | 9.63E−01 | 13.13 | 8.34E−49a | G6P transporter |
| TRPM7 | 0.94 | 9.22E−01 | 0.78 | 1.23E−01 | Non-SLC transporter of divalent cations |
P < 0.05.
FIG 8.
Top GO terms for genes enriched in human duodenal enteroids treated with 1,25(OH)2D3. (A) Crypt only; (B) villus only; (C) crypt and villus shared.
DISCUSSION
Vitamin D plays a major role in maintaining the integrity and function of the intestine as well as in the control of calcium homeostasis. However, the mechanisms by which 1,25(OH)2D3 regulates these processes are not fully understood. Our findings indicate the importance of the distal as well as the proximal segments of the intestine to understand the intestinal effects of 1,25(OH)2D3 and show that 1,25(OH)2D3-mediated responses involve epithelial cells in the crypts as well as the villus. This study also shows that the effects of 1,25(OH)2D3 on intestine are complex, are conserved in humans for active calcium uptake, and include calcium-regulating as well as calcium-independent effects in both proximal and distal intestine.
Previous investigations reported that 1,25(OH)2D3-mediated active calcium transport was localized only in the duodenum, where the most pronounced effects of 1,25(OH)2D3 during the active calcium transport process were induction of calbindin-D9k (S100g) and Trpv6 (4, 5, 9). Our current findings indicate that the classic 1,25(OH)2D3-activated genes (S100g and Trpv6) are also the two genes most markedly induced by 1,25(OH)2D3 in the distal intestine (similar to previous findings in the proximal intestine). In addition, our recent study showing, for the first time, a similar VDR-dependent calcium absorption efficiency between proximal colon and duodenum in the same mouse further establishes a role for the distal intestine in VDR-mediated intestinal calcium absorption (29). In that study, calcitriol glycosides and glucuronides, which target calcium absorption in the distal intestine, were also found to upregulate Trpv6 and S100g (the genes involved in active calcium transport) as well as calcium absorption in the colon. These results which provide direct in vivo evidence for the importance of VDR in the distal intestine suggest, together with earlier studies (11–14), that active calcium transport is a vitamin D-dependent process in the distal intestine, which plays a role in calcium homeostasis. Calcitriol glucuronide treatment may be a means to target calcitriol to the distal intestine and thus compensate for the loss of calcium absorption, for example, by gastric bypass surgery or small bowel resection. In the present investigation, specific genes previously associated with paracellular calcium transport were not significantly changed by 1,25(OH)2D3 in mouse distal intestine. However, Cldn2 was significantly induced by 1,25(OH)2D3 in the duodenum. Although it is possible that 1,25(OH)2D3 is involved in paracellular as well as active calcium transport, the physiological relevance of the reported regulation of genes by 1,25(OH)2D3 involved in paracellular transport remains to be determined (6, 7, 18).
Compared to that in other tissues, the highest levels of VDR are found in the small and large intestine. Studies related to 1,25(OH)2D3-mediated responses have focused on effects in differentiated enterocytes and have supported a role for 1,25(OH)2D3 in intestinal calcium absorption as well as barrier function (4–7). However, the effect of 1,25(OH)2D3 on intestinal stem cells has not been well investigated and has been a matter of debate (20–22). Previous studies using oral treatment reported that 1,25(OH)2D3 stimulates Cyp24a1 in intestinal epithelial cells at the villus or tip region and not at the crypts (20). Early microscopic autoradiographic studies, however, noted that tritium-labeled 1,25(OH)2D3 concentrated in both absorptive and crypt epithelial cells in the small and large intestines (30, 31). Using a highly specific and sensitive VDR antibody (D6) (32), we also noted, similar to in the autoradiographic studies, that VDR is present in villus and crypts. Our studies using isolated mouse villus and crypt as well as human enteroids with either a crypt-like phenotype (high proliferation, undifferentiated) or a villus-like phenotype (low proliferation, differentiated) identified 1,25(OH)2D3-induced target genes, including Cyp24a1, in both villus and crypts. The importance of vitamin D in the function of Lgr5+ stem cells, which are sensitive to Wnt stimulation and contribute to intestinal homeostatic regeneration, was previously noted by L. Augenlicht’s lab (22). They showed that inactivation of the VDR in mouse Lgr5+ intestinal crypt base columnar cells compromised stem cell functions of the Lgr5+ cells (22). Studies in human colon crypt organoids have also provided evidence that VDR is expressed in intestinal stem cells and has a regulatory role, as 1,25(OH)2D3 was found to induce stemness-related genes and to inhibit genes involved in cell proliferation (33). We are now only beginning to understand the regulatory role of vitamin D in intestinal stem cells (22, 33, 34). It is of interest that, in our in vivo studies in mice as well as in our studies using human enteroids, 1,25(OH)2D3 was found to regulate classic target genes in both villus and crypt. These findings suggest, similar to early studies showing the time dependency of 1,25(OH)2D3 action in enterocytes during their differentiation (35), that 1,25(OH)2D3 action in the intestine involves not only calcium absorption but also cellular differentiation and intestinal homeostasis. Although there have been many studies related to the intestinal effects of vitamin D in animals, ours is one of the few studies examining human vitamin D target genes. Previous studies have been conducted using human intestinal cell lines derived from colon (19, 36–38) as well as human biopsy specimens and duodenal explants (39, 40). The limitation of the studies in explants is the inability of the cells to renew as well as cell death in the explants. The enteroid culture system from human tissue allows growth and maintenance over time and provides an important in vitro model to examine both stem and non-stem cell responses to 1,25(OH)2D3. These epithelial cultures of the intestinal tract will provide technology enabling the verification of findings observed in animals and the identification of key components involved in 1,25(OH)2D3 action in human intestinal biology.
In addition to Cyp24a1, Trpv6, and S100g, Slc30a10, encoding a manganese efflux transporter, is also a 1,25(OH)2D3 target gene in both proximal and distal intestine. Slc30a10 is localized in the apical/luminal domain of the intestine, in liver, and in brain (41). Although initially thought to act as a Zn efflux transporter, recent studies have identified SLC30A10 as a mediator of Mn efflux which lowers cellular Mn levels and protects against Mn toxicity (23, 27, 42, 43). In clinical studies, patients carrying homozygous mutations in SLC30A10 show enhanced Mn levels, neurotoxicity, and liver injury (43, 44). It is of interest that Mn levels in the brain are minimally elevated in liver-specific Slc30a10 KO mice (41, 45). However, endoderm-specific KO mice (lacking Slc30a10 in liver and the gastrointestinal tract) were found to have markedly elevated Mn levels in the brain, indicating a critical role for the digestive system in the regulation of brain manganese (41, 45). Our results suggest that 1,25(OH)2D3, by inducing Slc30a10 in the intestine, may increase Mn excretion and thus may have therapeutic benefit without direct effects on the brain. Preliminary data comparing mice injected with MnCl2 (15 μg/g body weight) alone or MnCl2 plus 1,25(OH)2D3 (1 ng/g body weight) 3 times per week for 3 weeks showed that 1,25(OH)2D3 treatment results in decreased levels of blood Mn (control, 30 ± 2.3ng Mn/ml; MnCl2 treated, 403 ± 91ng Mn/ml; MnCl2 plus 1,25(OH)2D3 treated, 288 ± 3.3 ng Mn/ml) (S. Mukhopadhyay and S. Christakos, preliminary results). Further studies will be needed to determine effects of 1,25(OH)2D3 on neurotoxicity and on levels of Mn in different tissues as well as the effects of vitamin D deficiency, dietary calcium, and high-dose dietary vitamin D on Mn toxicity. Although elevated levels of Mn are neurotoxic, Mn is an essential element which plays a role in many cellular processes (46). With regard to bone homeostasis, it has been reported that the skeleton is positively influenced by Mn and that deficiency in Mn affects bone mass. Studies in rats have shown that Mn supplementation inhibits ovariectomy-induced bone loss (47). In addition, plasma levels of Mn were reported to be significantly decreased in groups of osteoporotic patients (48). More recent studies have noted that local manganese chloride treatment accelerates fracture healing in a rat model (49). However, excess of Mn can adversely affect bone quality. In our study using the Slc30a10 global KO mouse, which exhibits marked elevations in blood Mn, significant cortical bone abnormalities were noted. These findings further indicate the importance of maintaining Mn levels at an optimal range and not exceeding the levels required to accomplish essential functions. The mechanisms involved in the adverse effects on bone observed in the Slc30a10 KO mouse are not known. Whether the reduced thyroxine production observed in the Slc30a10KO mouse plays a role in the bone phenotype remains to be determined (50). The precise mechanism whereby Mn affects thyroxine biosynthesis is not known. It is of interest that a patient with a loss-of-function SLC30A10 mutation was also identified with hypothyroidism (51).
Due to the necessity of maintaining Mn levels in a narrow range in order to prevent toxicity, understanding the regulation of SLC30A10 is critical. The regulation of Slc30a10 in the intestine by 1,25(OH)2D3 suggests that 1,25(OH)2D3 not only affects absorption of calcium but also may affect the transport of other divalent cations. The time course of response to 1,25(OH)2D3 showed that expression of Trpv6 and Slc30a10 is significantly elevated 4 h after 1,25(OH)2D3. Twenty-four hours after administration of 1,25(OH)2D3, the expression of the genes for both transporters was decreased to levels observed prior to 0 h, consistent with a rapid response of both transporters to 1,25(OH)2D3, as previously noted for Trpv6 expression (52). The early induction of these transporters would be needed for rapid calcium uptake or Mn efflux. Although putative vitamin D response elements have been noted in the human and mouse genes which encode SLC30A10 (18, 19), studies are needed to determine VDR binding sites which are functionally linked to the expression of these genes. The significance of a possible inhibitory effect of unliganded VDR also requires further studies (53, 54).
To further understand the role of vitamin D in manganese homeostasis, the expression of the classic vitamin D target genes was examined in the intestine of the Slc30a10 KO mouse. In the Slc30a10 KO mouse, a marked decrease in the expression of Trpv6 and S100g in the duodenum, a primary route of manganese excretion (55), was consistently observed. These findings suggest that TRPV6, S100G, and SLC30A10 may work together in manganese efflux transport. Previous studies using HEK cells transfected with human (h) TRPV6 showed that TRPV6 is permeable to Mn as well as other heavy metal cations, indicating that TRPV6 isinvolved not only in calcium transport (56). Since intracellular calcium concentrations have been reported to correlate with the level of expression of TRPV6 (56), our findings of a decrease in Trpv6 (and S100g) in the intestine of mice with Slc30a10 deficiency and defective Mn excretion suggest the possibility of intracellular calcium-mediated regulation of intestinal Slc30a10. Kovacs et al. observed an inhibition of calcium uptake mediated by TRPV6 in the presence of high concentrations of Mn (56). Therefore, it is also possible that low levels of expression of the genes involved in calcium homeostasis are due to the high Mn levels in the Slc30a10 KO mice. Although not previously identified in vivo in the intestine, an interrelationship between calcium and Mn transport has been noted in other reports, including studies in mast cells, in mitochondria, and in brain (57–62). It has been suggested that Mn may interfere with brain calcium homeostasis (62). In the mitochondria, Mn transport has been reported to be activated by calcium (58, 60). However, calcium transport in the mitochondria is inhibited in the presence of Mn (58). In recent studies using HEK cells, it was noted that Slc30a10 extrusion of Mn was calcium dependent (63). The authors suggested that Slc30a10 uses a calcium gradient for active counter-ion exchange (63). Whether Mn efflux transport is regulated in part by calcium in the intestine (as suggested by our findings) and intestinal calcium transport is inhibited in the presence of high blood Mn levels (which are observed in the Slc30a10 KO mouse) remain to be determined. It is possible that the role of the distal intestine, where Slc30a10 is also induced by 1,25(OH)2D3, is compensatory when Mn efflux from the proximal intestine is disrupted. The relationship between calcium, the vitamin D endocrine system, and Mn efflux in the intestine is an unexplored topic. Since the digestive system has recently been identified as a critical regulator of Mn toxicity (41, 45), further studies are needed to provide new insight on the role of calcium andvitamin D on Mn homeostasis and protection against neurotoxicity.
Findings in our lab as well as prior reports have noted that 1,25(OH)2D3 treatment affects the gene expression of intestinal transporters in addition to Slc30a10. Expression of Slc37a2, which encodes a phosphate-linked glucose 6 phosphate antiporter, was previously shown to be induced by 1,25(OH)2D3 in mouse intestine (18). Although not regulated by 1,25(OH)2D3 in the mouse distal intestine, we found that SLC37A2 was induced by 1,25(OH)2D3 in human enteroids from duodenum (Table 2) and colon (data not shown). Regulation of SLC37A2 by 1,25(OH)2D3 is not specific to the intestine. SLC37A2 has also been suggested as a gene target and marker of vitamin D status in hematopoietic cells (64). Although SLC37A2 can transport glucose 6 phosphate (G6P), recent findings suggested that SLC37A2 does not have a role in the regulation of blood glucose (65). Since the functional role of SLC37A2 is not understood at this time, the physiological significance of regulation of gene expression by 1,25(OH)2D3 in both mouse and human intestines remains to be determined. Although we did not find that 1,25(OH)2D3 affected major Zn transporters, previous studies noted regulation of zinc transporters by 1,25(OH)2D3 in mouse intestine (18). Both calcium and zinc are known to play important roles in normal development and bone homeostasis (66–68). Early studies in chicks, however, noted that vitamin D or 1,25(OH)2D3 does not affect zinc absorption in zinc-deficient or -replete animals (69). Due to the importance of zinc as well as calcium for skeletal integrity, further studies are needed to determine an interrelationship between vitamin D and zinc and whether vitamin D has an effect on zinc homeostasis. In addition to regulation of SLC30A10 as well as SLC37A2, SLC34A2, encoding the intestinal cotransporter NaPi-IIb, was noted to be a gene target induced by 1,25(OH)2D3 in human enteroids derived from duodenum (SLC34A2 is expressed in the proximal intestine in humans and primarily in the ileum in mice). 1,25(OH)2D3 is known to stimulate intestinal phosphate absorption (70), and SLC34A2 has been reported to protect bone when dietary phosphate is restricted (71). However, the role of 1,25(OH)2D3 in the regulation of intestinal SLC34A2 (NaPi-IIb) has been a matter of debate (72–74). Further studies are needed to examine the regulation of this phosphate transporter in human intestine, its physiological significance in bone and phosphate homeostasis in humans, and whether it has a role in the dysregulation of phosphate observed in chronic kidney disease.
In a recent study, intestine-specific knockout of Trpm7, which encodes a channel kinase suggested to control magnesium levels, resulted in decreased serum zinc and calcium levels as well as decreased serum magnesium by postnatal day 5 (mice died by postnatal day 10) (25). The authors suggested that TRPM7 is the common gatekeeper for these ions in the intestine and that TRPM7 and not TRPV6 is the key factor in intestinal calcium absorption. However, these findings were observed in mice prior to the onset of vitamin D receptor-mediated active intestinal calcium absorption (which occurs at weaning). In addition, results of our transcriptomic analyses did not indicate regulation of Trpm7 by 1,25(OH)2D3 in the intestine of mice, and TRPM7 expression was unaffected by 1,25(OH)2D3 treatment in human enteroids. Thus, although TRPM7 is important in early postnatal life, TRPV6 and not TRPM7 is involved in the process of 1,25(OH)2D3-regulated intestinal calcium absorption.
In summary, our findings suggest the importance of the distal as well as the proximal intestinal segments in order to understand intestinal effects of vitamin D and show that 1,25(OH)2D3 effects involve intestinal epithelial cells in both the villus and crypt. In addition, our findings show direct transcriptomic responses to 1,25(OH)2D3 in human enteroids in both villus and crypt and that effects observed in mice are conserved in humans. Since SLC30A10 is the first reported transporter that uses a calcium gradient for active counter-ion exchange, our findings suggest that TRPV6, S100G, and SLC30A10 work together in Mn transport and that 1,25(OH)2D3 may have a role not only to maintain calcium homeostasis but also in the cellular homeostasis of other divalent ions.
MATERIALS AND METHODS
Animals.
Studies including the use of wild-type (C57BL/6J), KO/TG (mice expressing VDR only in the distal intestine), and Vdr knockout (KO) mice were approved by the Rutgers, New Jersey Medical School Animal Care and Use Committee. Mice were maintained in a virus and parasite-free barrier facility and exposed to a 12-h light/12-h dark cycle. Food and water were given ad libitum.
Transgenic mice expressing VDR only in the distal intestine were generated as previously described (14) using a 9.5-kb fragment from the CDX2 promoter, which directs transgene expression specifically to the distal intestine (75). The pBSKS-9.5 kb CDX2-human(h)VDR poly(A) transgene was injected into pronuclei of fertilized mouse oocytes at the Rutgers New Jersey Medical School Genome Editing Core Facility. Mice heterozygous for transgene integration and ablation of the endogenous VDR (TG+/− KO+/−) were bred to obtain mice with distal intestine-specific hVDR expression (TG/KO mice) (Fig. 1A). All TG/KO mice were fed standard rodent chow diet (Rodent Laboratory Chow 5001; Ralston Purina Co.) and were used at 12 to 14 weeks of age. Genotyping was performed by PCR using DNA extracted from tail biopsy specimens and mouse- and human-specific VDR primers (14). VDR KO mice were obtained from the Jackson Laboratory (originally from M. Demay, Harvard Medical School).
Findings in the TG/KO mouse intestine were compared to results obtained using vitamin D-deficient mice. To examine the response to 1,25(OH)2D3 in both TG/KO mice and vitamin D-deficient mice, each animal was administered three intraperitoneal injections of vehicle or 1,25(OH)2D3 (Cayman Chemical Company, Ann Arbor, MI) at a concentration of 1 ng/g body weight in a 9:1 mix of propylene glycol and ethanol at 48, 24, and 6 h before termination. The three-dose protocol was used to study short-term and long-term effects of 1,25(OH)2D3 administration.
The vitamin D-deficient mice were generated from C57BL/6J female mice fed a vitamin D deficient diet (−D) (0.47% Ca, 0.3% P; TD89123; Teklad diet from Envigo) for 3 to 4 weeks before mating and during pregnancy and lactation. Their offspring were provided the same diet starting directly after weaning until the tissues were harvested at 12 to 14 weeks of age.
Slc30a10 KO (Slc30a10−/−) mice were generated in the lab of Somshuvra Mukhopadhyay as previously described (27). Tissues from Slc30a10−/− mice were obtained at the University of Texas at Austin (UT Austin) under protocols approved by the Institutional Animal Care and Use Committee at UT Austin. Animals were fed regular rodent chow containing ∼84 μg Mn/g, and tissues were harvested at 5 to 6 weeks of age, as knockouts develop severe Mn toxicity beyond 8 weeks of age.
To compare the regulation of Slc30a10 to the regulation of classic 1,25(OH)2D3 target genes, a time course of response to 1,25(OH)2D3 as well as the response to high- or low-calcium diets and developmental changes in the expression of Slc30a10 were examined. The time course study was conducted using vitamin D-deficient mice injected with a single dose of 1,25(OH)2D3 (intraperitoneal [i.p.], 10 ng/g body weight) and killed at 1, 4, or 24 h after injection. The dietary study was conducted using 4-week-old mice fed either a high-calcium (1% Ca; TD92309) or low-calcium (0.02% Ca; TD86162) diet for 4 weeks. For the developmental study, timed pregnant, neonatal, and adult mice were fed a standard chow diet. Intestines were harvested from 18-day-old fetuses and 1-, 3-, and 6-week-old mice (n = 5 or 6 per group for 1-, 3-, and 6-week-old mice; for fetus, n = 3 pooled intestinal samples of 3 individual intestines). For the mouse studies, both male and female mice were studied. No sexual dimorphism was observed in the vitamin D target genes at these ages.
To determine whether classical as well as novel responses to 1,25(OH)2D3 occur in both villus and crypt, intestinal epithelial cells were isolated from both crypts and villi. The duodena from 3-month-old mice were washed in cold phosphate-buffered saline (PBS), cut into 1-cm pieces, and rotated in 3 mM EDTA in PBS at 4°C. After vigorous shaking to release the epithelium, crypts were depleted of contaminating villi by passing through a 70-μm cell strainer (BD Falcon, Tewksbury, MA). Villus epithelium was collected from the top of the 70-μm cell strainer (76). To test whether VDR target genes are conserved in humans, human enteroid cultures were used with either a crypt-like phenotype (i.e., high proliferation, undifferentiated cells) or a villus-like phenotype (i.e., low proliferation, differentiated cells). Human intestinal tissue specimens were obtained from endoscopy biopsies performed at Baylor College of Medicine (BCM) from adult patients of both sexes (3 female and 3 male; before endoscopy biopsies, informed consent was obtained from the patients, and the Institutional Review Board at BCM approved this study). Crypts were isolated using the EDTA chelation method described above. For human enteroids, crypts were resuspended in Matrigel and plated in a 24-well plate. Enteroids were maintained in complete medium with growth factors (CMGF+) with medium changes every 2 to 3 days (77). Enteroids were split every week by passing them through an insulin syringe and replated in Matrigel. For treatment of enteroids with 1,25(OH)2D3 or vehicle, enteroids were maintained in high-Wnt3A (undifferentiated) medium (CMGF+) or differentiation medium (CMGF+ without Wnt3A, nicotinamide, SB202, and R-Spondin) 3 days before treatment, with one medium change between (77). Subsequently, enteroids were treated with 100 nM 1,25(OH)2D3 (Cayman Chemical Company) or vehicle (ethanol) at equal volumes in medium. Twenty-four hours following treatment, enteroids were collected and processed.
RNA isolation and expression analysis.
Total RNA was isolated from mouse tissues, isolated mouse villus and crypt epithelia, and human organoids using RiboZol RNA extraction reagent (Amresco, Solon, OH) or TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions and subsequently purified with an RNeasy Plus universal kit (Qiagen, Hilden, Germany) using on-column DNase digestion (Qiagen). RNA concentration was measured with a NanoDrop spectrophotometer (ND-1000; Isogen Life Science, Utrecht, The Netherlands). RNA integrity was assessed using a denaturing agarose gel stained with ethidium bromide (EtBr) or by using a bioanalyzer nanochip (Agilent Technologies, Santa Clara, CA). For quantitative real-time PCR (qRT-PCR), 2 μg of total RNA was used to synthesize cDNA using a Superscript III first-strand synthesis system (Invitrogen) according to the manufacturer’s instructions. Relative quantification of target gene expression was performed using TaqMan analyses. TaqMan gene expression probes (Applied Biosystems, Foster City, CA) used for qRT-PCR were the following: Cyp24a1 (Mm00487244-m1), S100g (Mm00486654-m1), Slc30a1 (Mm00437377_m1), Slc30a10 (Mm00437377-m1), Slc37a2 (Mm00451435_m1), Trpv5 (Mm01166037_m1), Trpv6 (Mm00499069-m1), and Tjp1 (ZO-1 gene; Mm01320638_m1). Reactions were performed in a 7500 real-time PCR system (Applied Biosystems). The cycle steps were as followd: an initial 3-min incubation at 95°C, followed by 40 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s. Expression levels of genes of interest were normalized to Gapdh (Mm999999-g1). The comparative threshold cycle (2−ΔΔCT) method was used to calculate relative gene expression.
For global assessment of mouse RNA levels, total RNA was subjected to two rounds of poly(A) selection using oligo(dT)25 magnetic beads (New England Biolabs [NEB], Ipswich, MA). Illumina-compatible RNA-seq libraries were constructed using the NEBNext Ultra II RNA library prep with sample purification beads (catalog number no. E7775) and NEBNext Multiplex Oligos for Illumina (dual index primers set 1; catalog no. E7600) according to the manufacturer’s instructions. Poly(A) selection and library quality were assessed using TapeStation 2200 (Agilent Technologies, Santa Clara, CA), and libraries were quantified using Qubit 4.0 fluorometer (Thermo Fisher, Waltham, MA). The prepared libraries were sequenced on an Illumina NextSeq 500 instrument (Illumina, San Diego, CA). CLC Genomics Workbench version 11.0.1 (Qiagen, Venlo, Netherlands) was used for RNA-seq analysis. Demultiplexed fastq files from RNA-seq libraries were imported into the CLC software. Bases with low quality were trimmed, and reads were aligned to the Mus musculus reference genome build 9 (mm9) using Kallisto. The aligned reads were obtained using the RNA-seq analysis tool CLC Genomics Workbench. Kallisto (version 45) was utilized to quantify the transcript abundances of the RNA-seq samples through pseudoalignment, using single-end reads and an Ensembl mm9 transcriptome build index. The tximport (version 1.14.0) package (78) was run in R (version 3.6.2) to create gene level count matrices for use with DESeq2 (version 1.26) (79) by importing quantification data obtained from Kallisto. DESeq2 was then used to generate transcript levels in each tissue sample.
For global assessment of RNA levels from human enteroids samples, RNA-seq data were aligned to the reference human genome (hg38) using Kallisto. Subsequently, differential gene expressions for both mouse and human were assessed using DESeq2 in R, and transcript levels were obtained using a false-discovery rate of 0.05 and a cutoff 1.5-fold change.
For GO term analyses, transcript identifiers (IDs) were used with the biomaRt package (version 2.42) (80) along with RDavidWebService package (version 1.24) (81) as well as DAVID 6.8 (82) to identify biological processes, cellular components, and molecular functions in which input genes are enriched.
Western blot analysis.
For Western bolt analysis of VDR, total cellular protein was extracted with a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1.0% NP-40, and protease inhibitors. Protein content was measured using the Bradford assay (83) or a reducing agent- and detergent-compatible protein assay (Bio-Rad Laboratories, Inc., Hercules, CA). Fifty micrograms of denatured protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4% to 20% gradient gel; Bio-Rad Laboratories, Inc., Hercules, CA) and transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.) for Western blot analysis using an enhanced chemiluminescent detection system (Denville Scientific, Inc., Holliston, MA). β-Actin immunoblotting was used for sample normalization. Anti-VDR (D-6) (research resource identified [RRID], AB_628040) and anti-β-actin (RRID, AB_626632) as well as secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX).
Bone analysis.
μCT analysis of the left tibiae was performed ex vivo using a high-resolution SkyScan 1172 system (50 kV, 200 μA, 0.5-mm aluminum filter) to examine trabecular and cortical bone parameters (84). Serial tomographs, reconstructed from raw data using the cone-beam reconstruction software (NRecon, v.1.4.4.0; Skyscan), were used to compute trabecular and cortical parameters from the proximal metaphyseal and middiaphyseal areas, respectively. Analysis was performed according to the guidelines of the American Society for Bone and Mineral Research (85).
Serum analysis.
Serum calcium, assayed using a colorimetric assay (Pointe Scientific, Inc., Canton, MI), was determined by Heartland Laboratories, Ames, IA.
Statistical analysis.
Results are displayed as means ± standard errors of the means (SEMs). Data were analyzed using Student’s t test or analysis of variance (ANOVA) and additionally with Benjamini and Hochberg in a post hoc test to consider significant difference between groups (P < 0.05).
Data availability.
RNA-seq data from mouse tissues (GSE144978) and human enteroids (GSE159811) were deposited in the Gene Expression Omnibus of the National Center for Biotechnology.
ACKNOWLEDGMENTS
We thank M. Aburadi and A. Kyeremateng for assistance with certain aspects of this investigation.
S.L., S.C., S.M., N.F.S., M.P.V., and J.C.F. designed the study. S.L., J.D.L.C., S. Hutchens, Z.K.C., R.A., O.P.-C., J.H., P.D., and L.V. performed experiments. S.L., S.C., S.M., N.F.S., Z.K.C., M.P.V., J.C.F., G.C., L.V., P.D., P.S., and S. Husain provided interpretation of results. S.L. and S.C. wrote the paper with comments from all authors.
This study was supported by NIH grant DK112365 (to S.C., M.P.V., J.C.F., and N.F.S.) and NIH grant ESO24812 to S.M. as well as a grant from the FWO:G0A2416N to L.V. and G.C.
We disclose no conflicts of interest.
REFERENCES
- 1.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. 2016. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pike JW, Meyer MB, Benkusky NA, Lee SM, St John H, Carlson A, Onal M, Shamsuzzaman S. 2016. Genomic determinants of vitamin D-regulated gene expression. Vitam Horm 100:21–44. doi: 10.1016/bs.vh.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike JW, Christakos S. 2017. Biology and mechanisms of action of the vitamin D hormone. Endocrinol Metab Clin North Am 46:815–843. doi: 10.1016/j.ecl.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christakos S, Lieben L, Masuyama R, Carmeliet G. 2014. Vitamin D endocrine system and the intestine. Bonekey Rep 3:496. doi: 10.1038/bonekey.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christakos S 2012. Recent advances in our understanding of 1,25-dihydroxyvitamin D3 regulation of intestinal calcium absorption. Arch Biochem Biophys 523:73–76. doi: 10.1016/j.abb.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. 2008. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 19:1912–1921. doi: 10.1091/mbc.e07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kutuzova GD, Deluca HF. 2004. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D3 stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys 432:152–166. doi: 10.1016/j.abb.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasserman RH, Fullmer CS. 1995. Vitamin D and intestinal calcium transport: facts, speculations and hypotheses. J Nutr 125:1971S–1979S. doi: 10.1093/jn/125.suppl_7.1971S. [DOI] [PubMed] [Google Scholar]
- 9.Pansu D, Bellaton C, Roche C, Bronner F. 1983. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am J Physiol 244:G695–700. doi: 10.1152/ajpgi.1983.244.6.G695. [DOI] [PubMed] [Google Scholar]
- 10.Lee DB, Hu MS, Kayne LH, Nakhoul F, Jamgotchian N. 1991. The importance of non-vitamin D-mediated calcium absorption. Contrib Nephrol 91:14–20. doi: 10.1159/000420152. [DOI] [PubMed] [Google Scholar]
- 11.Lee DB, Walling MM, Levine BS, Gafter U, Silis V, Hodsman A, Coburn JW. 1981. Intestinal and metabolic effect of 1,25-dihydroxyvitamin D3 in normal adult rat. Am J Physiol 240:G90–G96. doi: 10.1152/ajpgi.1981.240.1.G90. [DOI] [PubMed] [Google Scholar]
- 12.Favus MJ, Kathpalia SC, Coe FL, Mond AE. 1980. Effects of diet calcium and 1,25-dihydroxyvitamin D3 on colon calcium active transport. Am J Physiol 238:G75–G78. doi: 10.1152/ajpgi.1980.238.2.G75. [DOI] [PubMed] [Google Scholar]
- 13.Auchere D, Tardivel S, Gounelle JC, Drueke T, Lacour B. 1998. Role of transcellular pathway in ileal Ca2+ absorption: stimulation by low-Ca2+ diet. Am J Physiol 275:G951–G956. [DOI] [PubMed] [Google Scholar]
- 14.Dhawan P, Veldurthy V, Yehia G, Hsaio C, Porta A, Kim KI, Patel N, Lieben L, Verlinden L, Carmeliet G, Christakos S. 2017. Transgenic expression of the vitamin D receptor restricted to the ileum, cecum, and colon of vitamin D receptor knockout mice rescues vitamin D receptor-dependent rickets. Endocrinology 158:3792–3804. doi: 10.1210/en.2017-00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. 2008. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294:G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 16.Sun J 2010. Vitamin D and mucosal immune function. Curr Opin Gastroenterol 26:591–595. doi: 10.1097/MOG.0b013e32833d4b9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbachano A, Fernandez-Barral A, Ferrer-Mayorga G, Costales-Carrera A, Larriba MJ, Munoz A. 2017. The endocrine vitamin D system in the gut. Mol Cell Endocrinol 453:79–87. doi: 10.1016/j.mce.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Lee SM, Riley EM, Meyer MB, Benkusky NA, Plum LA, DeLuca HF, Pike JW. 2015. 1,25-Dihydroxyvitamin D3 controls a cohort of vitamin D receptor target genes in the proximal intestine that is enriched for calcium-regulating components. J Biol Chem 290:18199–18215. doi: 10.1074/jbc.M115.665794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claro da Silva T, Hiller C, Gai Z, Kullak-Ublick GA. 2016. Vitamin D3 transactivates the zinc and manganese transporter SLC30A10 via the vitamin D receptor. J Steroid Biochem Mol Biol 163:77–87. doi: 10.1016/j.jsbmb.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds CJ, Koszewski NJ, Horst RL, Beitz DC, Goff JP. 2019. Localization of the 1,25-dihydroxyvitamin D-mediated response in the intestines of mice. J Steroid Biochem Mol Biol 186:56–60. doi: 10.1016/j.jsbmb.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colston KW, Mackay AG, Finlayson C, Wu JC, Maxwell JD. 1994. Localisation of vitamin D receptor in normal human duodenum and in patients with coeliac disease. Gut 35:1219–1225. doi: 10.1136/gut.35.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peregrina K, Houston M, Daroqui C, Dhima E, Sellers RS, Augenlicht LH. 2015. Vitamin D is a determinant of mouse intestinal Lgr5 stem cell functions. Carcinogenesis 36:25–31. doi: 10.1093/carcin/bgu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay S 2018. Familial manganese-induced neurotoxicity due to mutations in SLC30A10 or SLC39A14. Neurotoxicology 64:278–283. doi: 10.1016/j.neuro.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellett GL 2011. Alternative perspective on intestinal calcium absorption: proposed complementary actions of Ca(v)1.3 and TRPV6. Nutr Rev 69:347–370. doi: 10.1111/j.1753-4887.2011.00395.x. [DOI] [PubMed] [Google Scholar]
- 25.Mittermeier L, Demirkhanyan L, Stadlbauer B, Breit A, Recordati C, Hilgendorff A, Matsushita M, Braun A, Simmons DG, Zakharian E, Gudermann T, Chubanov V. 2019. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc Natl Acad Sci U S A 116:4706–4715. doi: 10.1073/pnas.1810633116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halloran BP, DeLuca HF. 1980. Calcium transport in small intestine during early development: role of vitamin D. Am J Physiol 239:G473–G479. doi: 10.1152/ajpgi.1980.239.6.G473. [DOI] [PubMed] [Google Scholar]
- 27.Hutchens S, Liu C, Jursa T, Shawlot W, Chaffee BK, Yin W, Gore AC, Aschner M, Smith DR, Mukhopadhyay S. 2017. Deficiency in the manganese efflux transporter SLC30A10 induces severe hypothyroidism in mice. J Biol Chem 292:9760–9773. doi: 10.1074/jbc.M117.783605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YG, Wu S, Sun J. 2013. Vitamin D, vitamin D receptor, and tissue barriers. Tissue Barriers 1:e23118. doi: 10.4161/tisb.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H, Horst RL, Koszewski NJ, Goff JP, Christakos S, Fleet JC. 2020. Targeting 1,25(OH)2D-mediated calcium absorption machinery in proximal colon with calcitriol glycosides and glucuronides. J Steroid Biochem Mol Biol 198:105574. doi: 10.1016/j.jsbmb.2019.105574. [DOI] [PubMed] [Google Scholar]
- 30.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. 1979. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science 206:1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 31.Stumpf WE 2008. Vitamin D and the digestive system. Eur J Drug Metab Pharmacokinet 33:85–100. doi: 10.1007/BF03191025. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Zhu J, DeLuca HF. 2012. Where is the vitamin D receptor? Arch Biochem Biophys 523:123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Barral A, Costales-Carrera A, Buira SP, Jung P, Ferrer-Mayorga G, Larriba MJ, Bustamante-Madrid P, Dominguez O, Real FX, Guerra-Pastrian L, Lafarga M, Garcia-Olmo D, Cantero R, Del Peso L, Batlle E, Rojo F, Munoz A, Barbachano A. 2020. Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J 287:53–72. doi: 10.1111/febs.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Peregrina K, Houston M, Augenlicht LH. 2020. Vitamin D and the nutritional environment in functions of intestinal stem cells: implications for tumorigenesis and prevention. J Steroid Biochem Mol Biol 198:105556. doi: 10.1016/j.jsbmb.2019.105556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu JC, Smith MW, Lawson DE. 1992. Time dependency of 1,25(OH)2D3 induction of calbindin mRNA and calbindin expression in chick enterocytes during their differentiation along the crypt-villus axis. Differentiation 51:195–200. doi: 10.1111/j.1432-0436.1992.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 36.Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. 2006. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20:1447–1461. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- 37.Wood RJ, Tchack L, Taparia S. 2001. 1,25-Dihydroxyvitamin D3 increases the expression of the CaT1 epithelial calcium channel in the Caco-2 human intestinal cell line. BMC Physiol 1:11. doi: 10.1186/1472-6793-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleet JC, Eksir F, Hance KW, Wood RJ. 2002. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol Gastrointest Liver Physiol 283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- 39.Walters JR, Balesaria S, Chavele KM, Taylor V, Berry JL, Khair U, Barley NF, van Heel DA, Field J, Hayat JO, Bhattacharjee A, Jeffery R, Poulsom R. 2006. Calcium channel TRPV6 expression in human duodenum: different relationships to the vitamin D system and aging in men and women. J Bone Miner Res 21:1770–1777. doi: 10.1359/jbmr.060721. [DOI] [PubMed] [Google Scholar]
- 40.Balesaria S, Sangha S, Walters JR. 2009. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am J Physiol Gastrointest Liver Physiol 297:G1193–G1197. doi: 10.1152/ajpgi.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor CA, Hutchens S, Liu C, Jursa T, Shawlot W, Aschner M, Smith DR, Mukhopadhyay S. 2019. SLC30A10 transporter in the digestive system regulates brain manganese under basal conditions while brain SLC30A10 protects against neurotoxicity. J Biol Chem 294:1860–1876. doi: 10.1074/jbc.RA118.005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leyva-Illades D, Chen P, Zogzas CE, Hutchens S, Mercado JM, Swaim CD, Morrisett RA, Bowman AB, Aschner M, Mukhopadhyay S. 2014. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J Neurosci 34:14079–14095. doi: 10.1523/JNEUROSCI.2329-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuschl K, Clayton PT, Gospe SM, Jr, Gulab S, Ibrahim S, Singhi P, Aulakh R, Ribeiro RT, Barsottini OG, Zaki MS, Del Rosario ML, Dyack S, Price V, Rideout A, Gordon K, Wevers RA, Chong WK, Mills PB. 2012. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet 90:457–466. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, Severijnen LA, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra BA, van de Warrenburg BP, Bonifati V. 2012. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet 90:467–477. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercadante CJ, Prajapati M, Conboy HL, Dash ME, Herrera C, Pettiglio MA, Cintron-Rivera L, Salesky MA, Rao DB, Bartnikas TB. 2019. Manganese transporter Slc30a10 controls physiological manganese excretion and toxicity. J Clin Invest 129:5442–5461. doi: 10.1172/JCI129710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen P, Bornhorst J, Aschner M. 2018. Manganese metabolism in humans. Front Biosci (Landmark Ed) 23:1655–1679. doi: 10.2741/4665. [DOI] [PubMed] [Google Scholar]
- 47.Rico H, Gomez-Raso N, Revilla M, Hernandez ER, Seco C, Paez E, Crespo E. 2000. Effects on bone loss of manganese alone or with copper supplement in ovariectomized rats. Eur J Obstet Gynecol Reprod Biol 90:97–101. doi: 10.1016/S0301-2115(99)00223-7. [DOI] [PubMed] [Google Scholar]
- 48.Heaney RP 1988. Nutritional factors in causation of osteoporosis. Ann Chir Gynaecol 77:176–179. [PubMed] [Google Scholar]
- 49.Hreha J, Wey A, Cunningham C, Krell ES, Brietbart EA, Paglia DN, Montemurro NJ, Nguyen DA, Lee YJ, Komlos D, Lim E, Benevenia J, O'Connor JP, Lin SS. 2015. Local manganese chloride treatment accelerates fracture healing in a rat model. J Orthop Res 33:122–130. doi: 10.1002/jor.22733. [DOI] [PubMed] [Google Scholar]
- 50.Liu C, Hutchens S, Jursa T, Shawlot W, Polishchuk EV, Polishchuk RS, Dray BK, Gore AC, Aschner M, Smith DR, Mukhopadhyay S. 2017. Hypothyroidism induced by loss of the manganese efflux transporter SLC30A10 may be explained by reduced thyroxine production. J Biol Chem 292:16605–16615. doi: 10.1074/jbc.M117.804989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anagianni S, Tuschl K. 2019. Genetic disorders of manganese metabolism. Curr Neurol Neurosci Rep 19:33. doi: 10.1007/s11910-019-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. 2003. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 53.Huet T, Laverny G, Ciesielski F, Molnar F, Ramamoorthy TG, Belorusova AY, Antony P, Potier N, Metzger D, Moras D, Rochel N. 2015. A vitamin D receptor selectively activated by gemini analogs reveals ligand dependent and independent effects. Cell Rep 10:516–526. doi: 10.1016/j.celrep.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 54.Lee SM, Pike JW. 2016. The vitamin D receptor functions as a transcription regulator in the absence of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 164:265–270. doi: 10.1016/j.jsbmb.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertinchamps AJ, Miller ST, Cotzias GC. 1966. Interdependence of routes excreting manganese. Am J Physiol 211:217–224. doi: 10.1152/ajplegacy.1966.211.1.217. [DOI] [PubMed] [Google Scholar]
- 56.Kovacs G, Danko T, Bergeron MJ, Balazs B, Suzuki Y, Zsembery A, Hediger MA. 2011. Heavy metal cations permeate the TRPV6 epithelial cation channel. Cell Calcium 49:43–55. doi: 10.1016/j.ceca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Fasolato C, Hoth M, Matthews G, Penner R. 1993. Ca2+ and Mn2+ influx through receptor-mediated activation of nonspecific cation channels in mast cells. Proc Natl Acad Sci U S A 90:3068–3072. doi: 10.1073/pnas.90.7.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavin CE, Gunter KK, Gunter TE. 1999. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology 20:445–453. [PubMed] [Google Scholar]
- 59.Kamer KJ, Sancak Y, Fomina Y, Meisel JD, Chaudhuri D, Grabarek Z, Mootha VK. 2018. MICU1 imparts the mitochondrial uniporter with the ability to discriminate between Ca2+ and Mn2+. Proc Natl Acad Sci U S A 115:E7960–E7969. doi: 10.1073/pnas.1807811115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vinogradov A, Scarpa A. 1973. The initial velocities of calcium uptake by rat liver mitochondria. J Biol Chem 248:5527–5531. [PubMed] [Google Scholar]
- 61.Ijomone OM, Aluko OM, Okoh COA, Martins AC, Jr, Aschner M. 2019. Role for calcium signaling in manganese neurotoxicity. J Trace Elem Med Biol 56:146–155. doi: 10.1016/j.jtemb.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Bornhorst J, Wehe CA, Huwel S, Karst U, Galla HJ, Schwerdtle T. 2012. Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J Biol Chem 287:17140–17151. doi: 10.1074/jbc.M112.344093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levy M, Elkoshi N, Barber-Zucker S, Hoch E, Zarivach R, Hershfinkel M, Sekler I. 2019. Zinc transporter 10 (ZnT10)-dependent extrusion of cellular Mn2+ is driven by an active Ca2+-coupled exchange. J Biol Chem 294:5879–5889. doi: 10.1074/jbc.RA118.006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilfinger J, Seuter S, Tuomainen TP, Virtanen JK, Voutilainen S, Nurmi T, de Mello VD, Uusitupa M, Carlberg C. 2014. Primary vitamin D receptor target genes as biomarkers for the vitamin D3 status in the hematopoietic system. J Nutr Biochem 25:875–884. doi: 10.1016/j.jnutbio.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Pan CJ, Chen SY, Jun HS, Lin SR, Mansfield BC, Chou JY. 2011. SLC37A1 and SLC37A2 are phosphate-linked, glucose-6-phosphate antiporters. PLoS One 6:e23157. doi: 10.1371/journal.pone.0023157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cousins RJ 2010. Gastrointestinal factors influencing zinc absorption and homeostasis. Int J Vitam Nutr Res 80:243–248. doi: 10.1024/0300-9831/a000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossi L, Migliaccio S, Corsi A, Marzia M, Bianco P, Teti A, Gambelli L, Cianfarani S, Paoletti F, Branca F. 2001. Reduced growth and skeletal changes in zinc-deficient growing rats are due to impaired growth plate activity and inanition. J Nutr 131:1142–1146. doi: 10.1093/jn/131.4.1142. [DOI] [PubMed] [Google Scholar]
- 68.Lieben L, Carmeliet G, Masuyama R. 2011. Calcemic actions of vitamin D: effects on the intestine, kidney and bone. Best Pract Res Clin Endocrinol Metab 25:561–572. doi: 10.1016/j.beem.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Koo SI, Fullmer CS, Wasserman RH. 1980. Effect to cholecalciferol and 1,25-dihydroxycholecalciferol on the intestinal absorption of zinc in the chick. J Nutr 110:1813–1818. doi: 10.1093/jn/110.9.1813. [DOI] [PubMed] [Google Scholar]
- 70.Williams KB, DeLuca HF. 2007. Characterization of intestinal phosphate absorption using a novel in vivo method. Am J Physiol Endocrinol Metab 292:E1917–E1921. doi: 10.1152/ajpendo.00654.2006. [DOI] [PubMed] [Google Scholar]
- 71.Knopfel T, Pastor-Arroyo EM, Schnitzbauer U, Kratschmar DV, Odermatt A, Pellegrini G, Hernando N, Wagner CA. 2017. The intestinal phosphate transporter NaPi-IIb (Slc34a2) is required to protect bone during dietary phosphate restriction. Sci Rep 7:11018. doi: 10.1038/s41598-017-10390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marks J 2019. The role of SLC34A2 in intestinal phosphate absorption and phosphate homeostasis. Pflugers Arch 471:165–173. doi: 10.1007/s00424-018-2221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katai K, Miyamoto K, Kishida S, Segawa H, Nii T, Tanaka H, Tani Y, Arai H, Tatsumi S, Morita K, Taketani Y, Takeda E. 1999. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem J 343 Pt 3:705–712. doi: 10.1042/0264-6021:3430705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, St-Arnoud R, Murer H, Biber J. 2005. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am J Physiol Cell Physiol 288:C429–C434. doi: 10.1152/ajpcell.00331.2004. [DOI] [PubMed] [Google Scholar]
- 75.Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, Cho KR, Fearon ER. 2007. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res 67:9721–9730. doi: 10.1158/0008-5472.CAN-07-2735. [DOI] [PubMed] [Google Scholar]
- 76.Chen L, Vasoya RP, Toke NH, Parthasarathy A, Luo S, Chiles E, Flores J, Gao N, Bonder EM, Su X, Verzi MP. 2020. HNF4 regulates fatty acid oxidation and is required for renewal of intestinal stem cells in mice. Gastroenterology 158:985.e9–999.e9. doi: 10.1053/j.gastro.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahe MM, Sundaram N, Watson CL, Shroyer NF, Helmrath MA. 2015. Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J Vis Exp 6:52483. doi: 10.3791/52483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soneson C, Love MI, Robinson MD. 2015. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4:1521. doi: 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durinck S, Spellman PT, Birney E, Huber W. 2009. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fresno C, Fernandez EA. 2013. RDAVIDWebService: a versatile R interface to DAVID. Bioinformatics 29:2810–2811. doi: 10.1093/bioinformatics/btt487. [DOI] [PubMed] [Google Scholar]
- 82.Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 83.Bradford MM 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 84.Lieben L, Benn BS, Ajibade D, Stockmans I, Moermans K, Hediger MA, Peng JB, Christakos S, Bouillon R, Carmeliet G. 2010. Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone 47:301–308. doi: 10.1016/j.bone.2010.04.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. 2010. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNA-seq data from mouse tissues (GSE144978) and human enteroids (GSE159811) were deposited in the Gene Expression Omnibus of the National Center for Biotechnology.