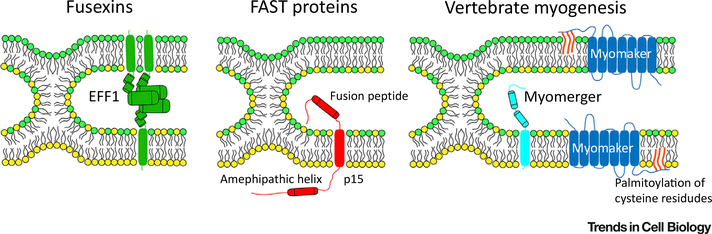
Figure 2. Diversity of Protein Machineries Driving Cell–Cell Fusion.
Representative fusogenic proteins from three classes are shown at the stage of the unstable hemifusion-stalk intermediate. EFF1, representing the fusexin family, acts in a trans-trimerization form to drive membrane fusion. The transmembrane domains perform a zippering-like action to bring opposing membranes together and drive pore formation. Fusion-associated small transmembrane (FAST) proteins act unilaterally to drive fusion of reovirus-infected cells. FAST proteins harness endogenous cellular machinery to promote cell adhesion. An extracellular fusion peptide inserts into the trans membrane, and an intracellular amphipathic helix is postulated to drive fusion pore formation. Myomaker and myomerger act independently at the membrane to drive a bipartite myoblast fusion mechanism. Myomaker is required for hemifusion to occur, and must be palmitoylated in its C-terminal region to function. Myomerger mediates pore formation through the activity of one or both extracellular helices.