Abstract
Study Objectives:
The association of mild obstructive sleep apnea (OSA) with important clinical outcomes remains unclear. We aimed to investigate the association between mild OSA and systemic arterial hypertension (SAH) in the European Sleep Apnea Database cohort.
Methods:
In a multicenter sample of 4,732 participants, we analyzed the risk of mild OSA (subclassified into 2 groups: mildAHI 5-<11/h (apnea-hypopnea index [AHI], 5 to <11 events/h) and mildAHI 11-<15/h (AHI, ≥11 to <15 events/h) compared with nonapneic snorers for prevalent SAH after adjustment for relevant confounding factors including sex, age, smoking, obesity, daytime sleepiness, dyslipidemia, chronic obstructive pulmonary disease, type 2 diabetes, and sleep test methodology (polygraphy or polysomnography).
Results:
SAH prevalence was higher in the mildAHI 11-<15/h OSA group compared with the mildAHI 5-<11/h group and nonapneic snorers (52% vs 45% vs 30%; P < .001). Corresponding adjusted odds ratios for SAH were 1.789 (mildAHI 11-<15/h; 95% confidence interval [CI], 1.49–2.15) and 1.558 (mildAHI 5-<11/h; 95%, CI, 1.34–1.82), respectively (P < .001). In sensitivity analysis, mildAHI 11-<15/h OSA remained a significant predictor for SAH both in the polygraphy (odds ratio, 1.779; 95% CI, 1.403–2.256; P < .001) and polysomnography groups (odds ratio, 1.424; 95% CI, 1.047–1.939; P = .025).
Conclusions:
Our data suggest a dose-response relationship between mild OSA and SAH risk, starting from 5 events/h in polygraphy recordings and continuing with a further risk increase in the 11- to <150-events/h range. These findings potentially introduce a challenge to traditional thresholds of OSA severity and may help to stratify participants with OSA according to cardiovascular risk.
Citation:
Bouloukaki I, Grote L, McNicholas WT, et al. Mild obstructive sleep apnea increases hypertension risk, challenging traditional severity classification. J Clin Sleep Med. 2020;16(6):889–898.
Keywords: European Sleep Apnea Database, mild obstructive sleep apnea, systemic arterial hypertension
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are data suggesting that mild obstructive sleep apnea (OSA) is associated with an increased risk of systemic arterial hypertension, although part of this association may be attributed to confounding factors. This study aimed to investigate the association between mild OSA and systemic arterial hypertension in the European Sleep Apnoea Database cohort.
Study Impact: Our results show a dose-response relationship between mild OSA and systemic arterial hypertension risk, starting from 5 events/h in polygraphy recordings and continuing with a further risk increase in the 11- to <15-events/h range. These findings potentially introduce an important challenge to the traditional thresholds of OSA severity and may also add to early identification of patients at risk.
INTRODUCTION
Mild obstructive sleep apnea (OSA) is a highly prevalent disorder in adults with potential adverse neurocognitive, metabolic, and cardiovascular complications.1 Changes in sleep recording technology, apnea-hypopnea scoring criteria, and the definition of sleep apnea according to the recent classification system (ICSD-3) have all contributed to an increased prevalence of OSA. In particular, mild OSA appears to provide the largest subgroup of this condition, accounting indifferent reports for up to 50–70% of all patients with OSA.2
Because mild OSA may progress to more severe disease, it is important to know whether this category is associated with adverse health outcomes, such as systemic arterial hypertension (SAH) and whether treatment improves these outcomes. However, there are conflicting data on this question. A recent research statement by the American Thoracic Society sought to quantify the impact of mild OSA in adults3 and concluded that there were no significant differences in the risk of SAH among patients with mild OSA when stratified by age, sex, body mass index (BMI), or daytime sleepiness. In contrast, a recent meta-analysis concluded that mild OSA is associated with an increased risk of SAH.4 Previous analysis from the European Sleep Apnoea Database (ESADA) cohort showed an independent association between OSA and SAH, possibly mediated by intermittent hypoxia during sleep.5 If mild OSA predicts prevalent SAH, then early identification and treatment of mild OSA assumes greater importance. Therefore, the aim of the current study was to investigate the relationship of mild OSA with SAH in the ESADA cohort and the role of various confounding factors in this relationship.
METHODS
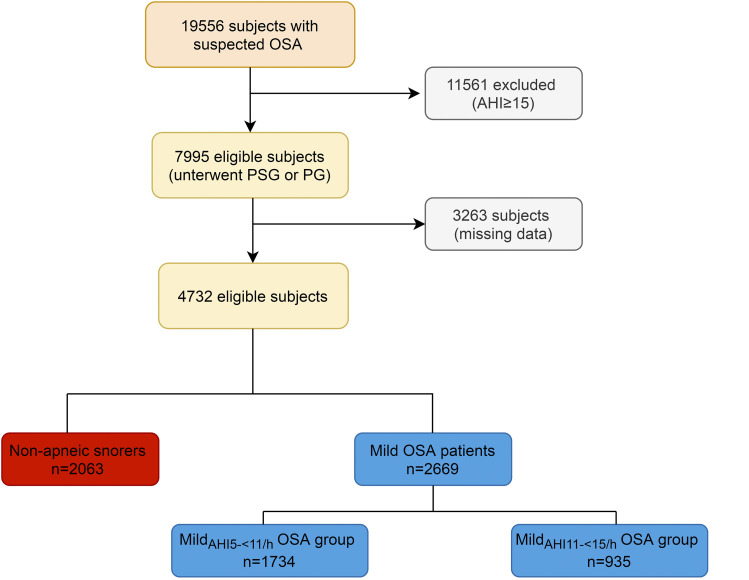
The ESADA cohort6 constitutes a collaborative European network project between mostly academic sleep centers. The ESADA cohort was established in 2007 with the objective to recruit a large prospective international cohort of treatment naïve participants with OSA. From the ESADA cohort, data recorded between March 2007 and May 2016 were analyzed. A cohort of 19,556 participants suspected of having OSA was assembled in 30 centers distributed across 20 countries in Europe and Israel. Inclusion criteria were as follows: referral because of suspected OSA, age of at least 18 years, and the ability to read, speak, and understand the local language. Exclusion criteria were as follows: previous treatment with continuous positive airway pressure or oral device, severe comorbidity unrelated to OSA with limited life expectancy, and alcohol or drug abuse within 1 year of inclusion. The flow chart of participants recruited is shown in Figure 1. Of 19,556 participants, 4,732 were analyzed. All participants signed an informed consent, and the study was approved by the local ethics committee of each participating center.
Figure 1. Flowchart of patients recruited.
Demographic characteristics and prevalent hypertension
The collected data included anthropometric parameters, such as age, sex, height, weight, BMI, neck circumference, waist/hip ratio, details of comorbidities (based on established diagnosis), and antihypertensive medication use and health-related lifestyle, such as smoking and alcohol consumption. Overweight and obese categories were defined according to the World Health Organization classification based on various BMI cutoffs. Medication use was determined according to the Anatomical Therapeutic Chemical classification system.7 In the current study, the diagnosis of SAH was based not only on the diagnosis provided by the referring physician obtained from medical records but also by the use of cardiovascular medications that were specifically used as antihypertensive medications. Blood pressure measurements were also taken in the sleep clinics according to hypertension guidelines,8,9 but the results were not used to define a hypertension status. The degree of self-reported daytime sleepiness is quantified by means of the Epworth Sleepiness Scale (ESS).10
Sleep data
Sleep apnea diagnosis and the classification of severity was assessed either by sleep polygraphy (PG; n = 3,136) or attended/unattended overnight polysomnography (PSG; n = 1,581 and 15, respectively) according to the prevailing clinical routine at each participating sleep center. A detailed analysis of the sleep apnea classification within the ESADA cohort has been published elsewhere.11 In short, the different polygraphic recording devices sampled data from a minimum of four channels (level 3 devices according to the American Academy of Sleep Medicine including flow [nasal pressure], respiratory effort, oxygen saturation),12 and the following parameters were determined: analyzed time, self-reported sleep time, apnea-hypopnea index (PG-AHI), oxygen desaturation index (PG-ODI), and mean/lowest arterial oxygen saturation. PSG studies were performed according to standard techniques with monitoring of electroencephalogram, electro-oculogram, electromyogram, flow (by oronasal thermistor and nasal air pressure transducer), thoracic and abdominal respiratory effort (by respiratory induction plethysmography), oximetry, and body position. All sleep recordings were manually interpreted over 30-second periods and analyzed according to the 2007 American Academy of Sleep Medicine scoring criteria.13 In particular, the recommended hypopnea scoring rule was adopted in almost 90% of PG analysis (a combination of a ≥30% reduction in airflow [compared with pre-event baseline] with a ≥4% reduction of oxygen saturation). For hypopnea definition in PSG recordings, most centers used the alternative scoring criteria (≥50% flow reduction associated with arousal or ≥3% reduction of oxygen saturation). AHI was calculated as the average number of apneas and hypopneas divided by the hours of total sleep time (in PSG) or of the sleep period (in PG), considered the estimated time between lights off and lights on. In PSG recordings, total sleep time, sleep efficiency (total sleep time/recording time), arousal index (AI), and sleep stages were also analyzed.
Nonapneic snorers (AHI < 5 events/h, n = 2,063) were defined as the reference category in the analysis. Mild OSA was subdivided into (1) participants with mildAHI5-<11 OSA (AHI, 5 to <11 events/h, n = 1,734) and (2) participants with mildAHI11-<15 OSA (AHI, ≥11 to <15 events/h, n = 935) to explore potential dose-response relationships even within the mild OSA range.
Statistical analysis
The primary sample consisted of 4,732 participants (Figure 1). Missing data for some covariates (missing or erroneous study variables, missing or incomplete sleep questionnaire data and/or missing medical history) reduced the sample size for multivariable models to a minimum of 4,200 participants. Results are presented as mean ± SD for continuous variables if normally distributed and as median (25th–75th percentile) if not. Qualitative variables are presented as absolute number (percentage). Variables that were normally distributed were compared among the 3 groups (nonapneic snorers, participants with mildAHI5-<11 OSA, and participants with mildAHI11-<15 OSA) using analysis of variance. If analysis of variance was significant, we used the Tukey-Kramer post hoc test to compare each pair. For variables that are not normally distributed, we used the Kruskal-Wallis test to compare the 3 groups. If the Kruskal-Wallis test was significant, Dunn’s pairwise tests were carried out for the 3 pairs of groups with adjustments for multiple testing using the Bonferroni correction. For categorical variables, we used χ2 to compare the 3 groups. Each of the 3 groups was examined for association with prevalent SAH. Nonapneic snorers (AHI, 0 to <5 events/h) served as the reference category for the computation of the effect size, and associations were quantified by computing relative prevalence odds ratios (ORs). All models were adjusted for the continuous variables like waist-to-hip ratio and neck circumference and also for the categorical variables including age > 60 years, sex, obesity, smoking status, type 2 diabetes mellitus, dyslipidemia, sleepiness, and chronic obstructive pulmonary disease. Age was considered categorically as age groups of 18–59 and ≥60 years, BMI as groups of obese (BMI ≥ 30 kg/m2) and nonobese (BMI < 30 kg/m2), and ESS as groups of participants who were sleepier (ESS ≥ 10) and participants who were not sleepy (ESS < 10). We refer to these models as full models. Next, to test the robustness of covariate selection, we used forward stepwise selection procedures to identify parsimonious models of the covariates. Results were considered significant when P < .05. Data were analyzed using SPSS software (version 25, SPSS Inc, Chicago, IL).
RESULTS
Sample characteristics
Descriptive characteristics of the study population and the distribution of covariates are shown in Table 1. Individuals in the mildAHI11-<15 OSA group were older, more obese, were more frequently males, and had higher prevalence of comorbidities compared with nonapneic snorers and participants with mildAHI5-<11 OSA. However, the 3 groups did not differ significantly in terms of self-reported daytime sleepiness.
Table 1.
Clinical characteristics in participants with mild OSA and nonapneic snorers.
| Total Population (N = 4,732) | Total Population According to AHI | ||||
|---|---|---|---|---|---|
| Nonapneic Snorers (AHI < 5 events/h) (n = 2,063) | Patients with MildAHI 5-<11/h OSA (AHI, 5 to <11 events/h) (n = 1,734) | Patients with MildAHI 11-<15/h OSA (AHI, 11 to ≤15 events/h) (n = 935) | P Across All | ||
| Demographics | |||||
| Sex, males | 2,579 (55%) | 996 (48%) | 991 (57%)b | 592 (63%)a,c | <.001 |
| Age (y) | 52.4 ± 12.5 | 49.5 ± 13.0 | 54.3 ± 11.8b | 55.5 ± 11.2a,c | <.001 |
| Age ≥ 60 y | 1,448 (31%) | 492 (24%) | 592 (34%)b | 364 (39%)a,c | <.001 |
| BMI (kg/m2) | 29.8 ± 6.1 | 28.9 ± 5.8 | 30.4 ± 6.3b | 30.5 ± 6.0a | <.001 |
| BMI ≥ 30 kg/m2 | 1976 (42%) | 744 (36%) | 789 (46%)b | 443 (48%)a | <.001 |
| NC (cm) | 39.4 ± 4.2 | 38.6 ± 4.1 | 39.9 ± 4.1b | 40.4 ± 4.0a,c | <.001 |
| WC (cm) | 102.6 ± 14.7 | 99.8 ± 14.8 | 104.6 ± 14.5b | 105.4 ± 13.7a | <.001 |
| HC (cm) | 108.2 ± 12.1 | 107.1 ± 11.9 | 109.3 ± 12.6b | 108.7 ± 11.7a | <.001 |
| Waist-to-hip ratio | 0.95 ± 0.09 | 0.93 ± 0.09 | 0.96 ± 0.08b | 0.97 ± 0.08a,c | <.001 |
| Smoking status | |||||
| Never | 3,641 (77%) | 1,550 (75%) | 1,337 (88%) | 754 (81%)a,c | |
| Current/former | 1,069 (23%) | 511 (25%) | 384 (22%) | 174 (19%)a,c | .001 |
| Alcohol, units/w | 0.5 (0–4) | 1 (0–4) | 0.5 (0–4) | 0 (0–4)a | .03 |
| SBP | 133.6 ± 18.6 | 131.8 ± 12.6 | 134.7 ± 18.8b | 135.5 ± 17.9a | <.001 |
| DBP | 81.8 ± 11.4 | 81.4 ± 11.4 | 82.3 ± 11.7 | 81.7 ± 10.9 | .07 |
| Sleep-disordered breathing | |||||
| AHI | 6.3 ± 4.3 | 2.2 ± 1.4 | 7.8 ± 1.7b | 12.8 ± 1.2a,c | <.001 |
| ODI | 4.4 (1.7–8.5) | 1.9 (0.7–3.7) | 6.4 (3.9–9.1)b | 10.3 (6.6–13.7)a,c | <.001 |
| Mean oxygen saturation | 94.5 (93.1–95.7) | 95 (93.6–96.0) | 94.1 (93.0–95.2)b | 94.1 (93.0–95.2)a | <.001 |
| Lowest oxygen saturation | 87 (83–90) | 89 (86–91) | 86 (82–88)b | 84 (81–88)a,c | <.001 |
| Daytime sleepiness | |||||
| ESS | 9.3 ± 5.2 | 9.3 ± 5.3 | 9.2 ± 5.0 | 9.3 ± 5.0 | .82 |
| ESS ≥ 10 | 2,129 (47%) | 947 (47%) | 773 (46%) | 409 (46%) | .86 |
| Comorbidities | |||||
| SAH | 1,878 (40%) | 610 (30%) | 784 (45%)b | 483 (52%)a,c | <.001 |
| Diabetes type 2 | 585 (12%) | 202 (10%) | 231 (13%)b | 152 (16%)a,c | <.001 |
| Hyperlipidemia | 951 (20%) | 338 (16%) | 379(22%)b | 234 (25%)a | <.001 |
| COPD | 297 (6%) | 131 (6%) | 108 (6%) | 58 (6%) | .98 |
| Coronary heart disease | 383 (8%) | 128 (6%) | 154 (9%)b | 101 (11%)a | <.001 |
| Cerebrovascular disease | 125 (3%) | 34 (2%) | 64 (4%)b | 27 (3%)a | <.001 |
| Atrial fibrillation | 110 (2%) | 37 (2%) | 37 (3%) | 26 (3%) | .11 |
Data are presented as mean values ± SD, median (25th–75th percentile), or n (%). aP < .05 mildAHI 11-<15/h vs nonapneic snorers. bP < .05 mildAHI 5-<11/h vs nonapneic snorers. cP < .05 patients with mildAHI 11-<15/h vs mildAHI 5-<11/h OSA. AHI = apnea-hypopnea index, BMI = body mass index, COPD = chronic obstructive pulmonary disease, DBP = diastolic blood pressure, ESS = Epworth sleepiness scale, HC = hip circumference, NC = neck circumference, ODI = oxygen desaturation index, SAH = systemic arterial hypertension, SBP = systolic blood pressure, WC = waist circumference.
Effect of mild OSA on prevalent hypertension
Unadjusted systolic blood pressure and percentage of SAH diagnosis were higher in the mildAHI11-<15 OSA group compared with the mildAHI5-<11 group and nonapneic snorers (Table 1). A higher percentage in the whole mild OSA population used at least 2 antihypertensive drugs compared with nonapneic snorers (28% vs 16%; P < .001).
Mild OSA predicted prevalent SAH in all models. The mildAHI11-<15 OSA group had the highest OR for prevalent hypertension (Table 2), suggesting a dose-response relationship between OSA severity and SAH prevalence even within the AHI spectrum of mild OSA. Multiple stepwise logistic regression analysis also showed that age ≥ 60 years, waist-to-hip ratio, type 2 diabetes mellitus, and hyperlipidemia were significantly associated with higher odds for SAH (Table 3).
Table 2.
Crude and adjusted odds ratios for the relationship of prevalent systemic arterial hypertension and the mild OSA groups.
| Model | Total (n = 4,732) | B | OR (95% CI) | P |
|---|---|---|---|---|
| Model 0 | ||||
| MildAHI 5-<11/h OSA | 4,732 | 0.674 | 1.961 (1.716–2.242) | <.001 |
| MildAHI 11-<15/h OSA | 4,732 | 0.932 | 2.539 (2.165–2.978) | <.001 |
| Model 1 | ||||
| MildAHI 5-<11/h OSA | 4,200 | 0.444 | 1.558 (1.337–1.816) | <.001 |
| MildAHI 11-<15/h OSA | 4,200 | 0.582 | 1.789 (1.487–2.152) | <.001 |
| Model 2 | ||||
| MildAHI 5-<11/h OSA | 4,323 | 0.458 | 1.580 (1.360–1.837) | <.001 |
| MildAHI 11-<15/h OSA | 4,323 | 0.582 | 1.790 (1.496–2.142) | <.001 |
Model 0, unadjusted; model 1, full model included the covariates age > 60 years, sex, body mass index ≥ 30 kg/m2, waist/hip ratio, neck circumference, type 2 diabetes mellitus, dyslipidemia, chronic obstructive pulmonary disease, smoking status, Epworth Sleepiness Scale score ≥ 10; model 2, parsimonious model excluded from the full model the covariates sex, smoking, COPD, and Epworth Sleepiness Scale score ≥ 10. CI = confidence interval, COPD = chronic obstructive pulmonary disease, OR = odds ratio, OSA = obstructive sleep apnea.
Table 3.
Multiple stepwise logistic regression analysis of the relationship between systemic arterial hypertension and various independent variables.
| Variable | B | SE | P | OR (95% CI) |
|---|---|---|---|---|
| MildAHI 5-<11/h OSA | 0.444 | 0.078 | <.001 | 1.558 (1.337–1.816) |
| MildAHI 11-<15/h OSA | 0.582 | 0.094 | <.001 | 1.789 (1.487–2.152) |
| Females vs males | 0.300 | 0.100 | .003 | 1.350 (1.110–1.643) |
| Age ≥ 60 y | 0.858 | 0.077 | <.001 | 2.358 (2.027–2.743) |
| BMI ≥ 30 kg/m2 | 0.197 | 0.087 | .023 | 1.218 (1.028–1.444) |
| Waist/hip ratio | 2.102 | 0.529 | <.001 | 8.184 (2.903–23.074) |
| Neck circumference | 0.073 | 0.013 | <.001 | 1.076 (1.048–1.105) |
| ESS score ≥ 10 | −0.278 | 0.070 | <.001 | 0.757 (0.660–0.869) |
| Smoking | −0.377 | 0.087 | <.001 | 0.686 (0.579–0.813) |
| Type 2 diabetes | 0.734 | 0.108 | <.001 | 2.084 (1.685–2.577) |
| Dyslipidemia | 0.721 | 0.085 | <.001 | 2.056 (1.742–2.428) |
| COPD | −0.291 | 0.142 | .041 | 0.748 (0.566–0.988) |
BMI = body mass index, CI = confidence interval, COPD = chronic obstructive pulmonary disease, ESS = Epworth Sleepiness Scale, OR = odds ratio, OSA = obstructive sleep apnea.
We also explored other measures of OSA activity as predictors for SAH. In the full adjusted models, ODI as continuous (OR, 1.030; 95% confidence interval [CI], 1.018–1.043; P < .001) or categorical value (Table 4), mean SaO2 (OR, 0.945; 95% CI, 0.912–0.980; P = .002), and lowest SaO2 (OR, 0.968; 95% CI, 0.957–0.980; P < .001) predicted SAH. However, in models with mild OSA and ODI groups (ODI, 5 to <11 and 11 to <15 events/h) entered simultaneously, only mild OSA groups remained significant predictors of SAH (Table 4).
Table 4.
Crude and adjusted odds ratios for prevalent systemic arterial hypertension in models containing either mild OSA groups or ODI as categorical variables (ODI 5-<11 and 11-<15) entered separately or simultaneously.
| Model | No. of Participants | B | OR (95% CI) | P |
|---|---|---|---|---|
| Mild OSA or ODI groups entered separately | ||||
| MildAHI 5-<11/h OSA | ||||
| Model 0 | 4,732 | 0.674 | 1.961 (1.716–2.242) | <.001 |
| Model 1 | 4,200 | 0.444 | 1.558 (1.337–1.816) | <.001 |
| Model 2 | 4,323 | 0.458 | 1.580 (1.360–1.837) | <.001 |
| MildAHI 11-<15/h OSA | ||||
| Model 0 | 4,732 | 0.932 | 2.539 (2.165–2.978) | <.001 |
| Model 1 | 4,200 | 0.582 | 1.789 (1.487–2.152) | <.001 |
| Model 2 | 4,323 | 0.582 | 1.790 (1.496–2.142) | <.001 |
| ODI 5–<11 events/h | ||||
| Model 0 | 4,332 | 0.703 | 2.020 (1.766–2.311) | <.001 |
| Model 1 | 3,858 | 0.411 | 1.509 (1.291–1.764) | <.001 |
| Model 2 | 3,967 | 0.439 | 1.551 (1.331–1.806) | <.001 |
| ODI 11–<15 events/h | ||||
| Model 0 | 4,332 | 0.876 | 2.401 (1.957–2.946) | <.001 |
| Model 1 | 3,858 | 0.381 | 1.464 (1.156–1.854) | .002 |
| Model 2 | 3,967 | 0.416 | 1.515 (1.203–1.909) | <.001 |
| Both mild OSA and ODI groups entered simultaneously | ||||
| MildAHI 5-<11/h OSA | ||||
| Model 0 | 4,332 | 0.419 | 1.521 (1.300–1.780) | <.001 |
| Model 1 | 3,858 | 0.345 | 1.412 (1.181–1.617) | <.001 |
| Model 2 | 3,967 | 0.348 | 1.416(1.189–1.686) | <.001 |
| MildAHI 11-<15/h OSA | ||||
| Model 0 | 4,332 | 0.523 | 1.686(1.372–2.073) | <.001 |
| Model 1 | 3,858 | 0.461 | 1.586 (1.252–2.007) | <.001 |
| Model 2 | 3,967 | 0.426 | 1.531(1.217–1.926) | <.001 |
| ODI 5–<11 events/h | ||||
| Model 0 | 4,332 | 0.471 | 1.602 (1.372–1.871) | <.001 |
| Model 1 | 3,858 | 0.217 | 1.243 (1.039–1.486) | .017 |
| Model 2 | 3,967 | 0.248 | 1.281 (1.075–1.526) | .006 |
| ODI 11–<15 events/h | ||||
| Model 0 | 4,332 | 0.555 | 1.741 (1.377–2.202) | <.001 |
| Model 1 | 3,858 | 0.099 | 1.104 (0.842–1.448) | .474 |
| Model 2 | 3,967 | 0.151 | 1.163 (0.882–1.515) | .265 |
CI = confidence interval, ODI = oxygen desaturation index, OR = odds ratio, OSA = obstructive sleep apnea.
Role of sleep studies used (PG vs PGS) on SAH prevalence
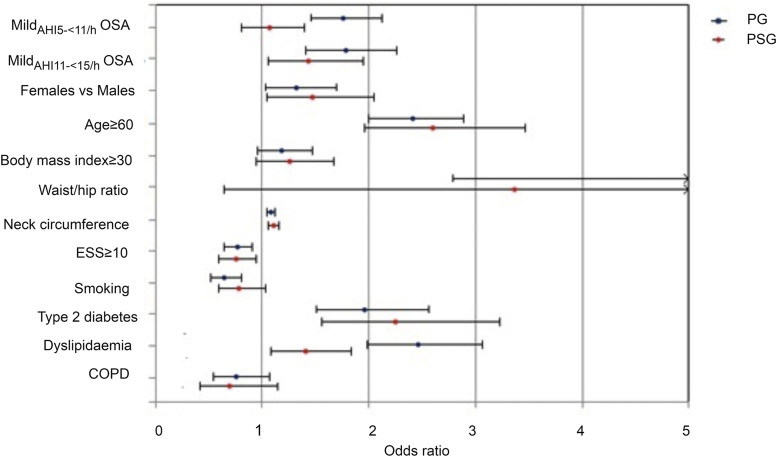
The type of sleep study used for the diagnosis of mild OSA (PSG or PG) was found to influence the predictive value of the 2 mild OSA groups on prevalent SAH. MildAHI 5-<11/h OSA was a significant predictor in the PG group (P < .001) but not in the PSG group (P = .679; Figure 2). However, mildAHI 11-<15/h OSA remained a significant predictor in the PG (P < .001) and PSG groups (P = .025; Table 5).
Figure 2. Multiple logistic regression analysis of the relationship between systemic arterial hypertension and mild OSA groups according to type of study (polygraphy or polysomnography).
Error bars indicate 95% confidence intervals. COPD = chronic obstructive pulmonary disease, ESS = Epworth Sleepiness Scale, OSA = obstructive sleep apnea.
Table 5.
Crude and adjusted odds ratios for prevalent systemic arterial hypertension in models containing the mild OSA groups, according to sleep study used.
| Total | B | OR (95% CI) | P | |
|---|---|---|---|---|
| PG (n = 3,135) | ||||
| Model 0 | ||||
| MildAHI 5-<11/h OSA | 3,135 | 0.827 | 2.285 (1.939–2.694) | <.001 |
| MildAHI 11-<15/h OSA | 3,135 | 1.022 | 2.780 (2.261–3.418) | <.001 |
| Model 1 | ||||
| MildAHI 5-<11/h OSA | 2,869 | 0.563 | 1.757 (1.457–2.118) | <.001 |
| MildAHI 11-<15/h OSA | 2,869 | 0.576 | 1.779 (1.403–2.256) | <.001 |
| Model 2 | ||||
| MildAHI 5-<11/h OSA | 2,921 | 0.567 | 1.762 (1.470–2.124) | <.001 |
| MildAHI 11-<15/h OSA | 2,921 | 0.569 | 1.769 (1.395–2.226) | <.001 |
| PSG (n = 1,596) | ||||
| Model 0 | ||||
| MildAHI 5-<11/h OSA | 1,596 | 0.306 | 1.358 (1.076–1.714) | .010 |
| MildAHI 11-<15/h OSA | 1,596 | 0.645 | 1.906 (1.474–2.465) | <.001 |
| Model 1 | ||||
| MildAHI 5-<11/h OSA | 1,330 | 0.058 | 1.060 (0.805–1.396) | .679 |
| MildAHI 11-<15/h OSA | 1,330 | 0.354 | 1.424 (1.047–1.939) | .025 |
| Model 2 | ||||
| MildAHI 5-<11/h OSA | 1,401 | 0.118 | 1.125 (0.862–1.489) | .387 |
| MildAHI 11-<15/h OSA | 1,401 | 0.389 | 1.476 (1.100–1.981) | .009 |
CI = confidence interval, OR = odds ratio, OSA = obstructive sleep apnea, PG = polygraphy, PSG = polysomnography.
Subgroup analysis
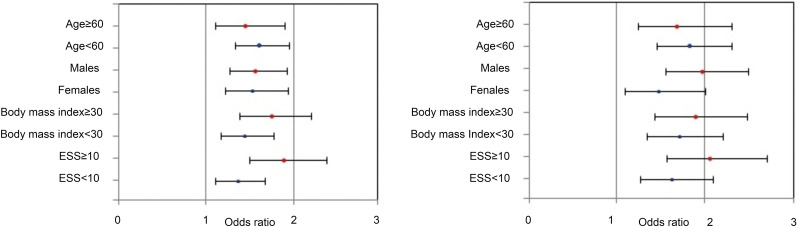
Further analysis demonstrated that mild OSA predicted prevalent SAH in all age, BMI, and ESS groups and sexes (all P < .05), with slightly stronger associations observed in younger adults (<60 years), males, participants who were sleepier (ESS ≥ 10), and participants with obesity (BMI ≥ 30 kg/m2; Figure 3).
Figure 3. Multiple logistic regression analysis of the relationship between systemic arterial hypertension and mild OSA groups according to age, sex, BMI, and ESS.
Left: AHI 5 to < 11 events/h. Right: AHI 11 to < 15 events/h. Error bars indicate 95% confidence intervals. BMI = body mass index, ESS = Epworth Sleepiness Scale, OSA = obstructive sleep apnea.
Because multiple regression models cannot completely remove confounding effects, we performed a subanalysis excluding participants with preexisting cardiovascular or cerebrovascular diseases (ie, stroke, angina), diabetes, and hyperlipidemia, confirming the previous results in the whole group and in PG and PSG groups separately (Table S1, Table S2, and Table S3 in the supplemental material).
Finally, to explore the role of sleep fragmentation on SAH risk, we performed a subanalysis in 685 participants who underwent PSG with available data on AI. Although mildAHI 5-<11/h and mildAHI 11-<15/h OSA groups showed increased AI (Table S4) compared with nonapneic snorers (19 vs 14 and 15 vs 14, respectively; P < .001), AI was not a significant predictor of SAH (OR, 0.989; 95% CI, 0.973–1.004; P = .155) after adjustment for confounders. Similar results were obtained when different AI cutoffs (>10, >20) were used as categorical variables (all P > .05).
DISCUSSION
This study suggests that mild OSA was associated with a 56% (AHI 5 to <11 events/h) and 79% (AHI 11 to <15 events/h) risk increase of SAH, and the association was slightly stronger in participants who were younger, obese, sleepy, and male. Importantly, the OSA-mediated risk for SAH remained after extensive adjustment for potential confounders including measures of intermittent hypoxia.
It is known that approximately half of all patients with OSA present with comorbid SAH.14 However, most published studies on OSA have focused on patients with moderate or severe disease, and there is a paucity of studies selectively assessing patients with the mild form of the disease. The possible association between mild OSA and SAH has attracted considerable attention in recent years.3,4 Indeed, the risk for incident hypertension was significantly increased in mild OSA in prospective cohort studies,15,16 and there was an increased prevalence of a nondipping blood pressure pattern in patients with mild OSA.17 A recent meta-analysis also found that across all OSA severities, there was an association with SAH in a dose-dependent manner.4 A statement from the American Thoracic Society3 suggested that despite the compelling evidence of a causal relationship between these 2 conditions,18,19 part of this association may be attributed to confounding factors.20–24 We therefore performed extensive adjustment in our statistical models including comorbidities, several measures of obesity, and different indices of OSA severity.
In clinical research, mild OSA has almost universally been defined as an AHI in the range of 5–15 events/h. In our study, 2 cutoff limits of AHI were applied, attempting to further identify subphenotypes of patients at risk within the mild OSA population. Based on these AHI severity criteria, the mildAHI 5-<11/h OSA group (AHI 5 to <11 events/h) remained a significant predictor for SAH when the sleep study was performed with PG. This information is of particular importance as a large proportion of European patients are diagnosed using PG without application of AHI thresholds for OSA severity derived from PG cohorts.25 Furthermore, the mildAHI 11-<15/h OSA group with AHI ≥11 to <15 events/h was the stronger predictor of SAH in both the PG and PSG groups. Therefore, this study provided novel data that may challenge the conventional threshold defining mild OSA independently of the sleep study methodology used as AHI >5 to <15 events/h in relation to SAH risk.
A strength of our study is the large number of participants included and the multicentric, multinational setting, which increases the generalizability of our findings. In contrast, certain limitations of our study need to be considered. First, participants were enrolled based on a clinical referral to a sleep center, and they do not represent the general population. Second, given that our study was cross-sectional, we cannot assign directionality to the associations between mild OSA and SAH. Also, the duration of OSA is difficult to ascertain in this population, and the cumulative exposure to OSA may vary considerably among participants. Another potential limitation is the lack of 24-hour ambulatory blood pressure monitoring measurements, which could provide nocturnal blood pressure measurements as an additional variable of assessing SAH and its control in these patients. Finally, study participants represented a mixture of individuals undergoing PG or PSG investigations according to clinical routine in the participating centers, and no central scoring of events has been applied. We showed previously in the ESADA cohort,11 as have other investigators elsewhere,26,27 that PG sleep studies will underestimate AHI, and this discrepancy is likely to be most evident in mild OSA, whereas in severe disease (AHI > 30 events/h), small discrepancies in the AHI will have marginal practical implications for disease classification and management. Furthermore, this underdiagnosis and possible misclassification of OSA need to be taken into account because a large proportion of European patients, as in our study, are diagnosed using PG without application of different AHI thresholds for OSA severity.25 We therefore performed sensitivity analysis separately in the PG and PSG groups, which confirmed the main study results and adds important new insights.
Our findings have multiple clinical implications. First, we illustrated an AHI level of ≥11 events/h was associated with a steep increase in the odds for SAH. This may lead to early identification of patients at risk and treatment despite the lack of other daytime or sleep-related symptoms. Second, considering that OSA has a tendency to progress from mild sleep-disordered breathing to more severe OSA over a varying period of time,28,29 early intervention with personalized treatment and reduction of risk factors should be initiated.30–32 Third, our data clearly emphasize the need for outcome-based thresholds of OSA severity, which take the sleep diagnostic method into account. The risk impact at a lower AHI value was shown in the mild OSA group assessed by PG. The result needs to be verified by other studies before new definitions of mild, moderate, and severe sleep apnea based on PG recordings should be applied.
In conclusion, our findings suggest that mild OSA is an independent risk factor for SAH, mainly in the 11- to <15 events/h range of AHI. These findings potentially introduce an important challenge to the traditional thresholds of OSA severity and may also add to early identification of patients at risk.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. L. Grote received grants from Bayer, Resmed, Respironics/Philips, and from the European Respiratory Society during the conduct of the study; nonfinancial support from Itamar Medical, Resmed, Philips, and Astra Zeneca, outside the submitted work; and has been issued a patent on sleep apnea therapy. J. Hedner received grants from ResMed, Respironics, and Bayer on behalf of the European Sleep Apnoea Database International Study Group; received speaker fees from ResMed, Itamar, Merck, and Jazz Pharmaceuticals outside the submitted work; and has been issued a patent on sleep apnea therapy. J. Verbraecken received grants and personal fees from ResMed, personal fees from Philips, personal fees from Sanofi, personal fees from Agfa-Gevaert, grants and personal fees from Bioprojet, grants and personal fees from Jazz Pharmaceutics, grants from AirLiquide, personal fees from Springer, grants from Westfalen Medical, grants from SomnoMed, grants from Vivisol, grants from Total Care, grants from Medidis, grants from Fisher & Paykel, grants from Wave Medical, grants from OSG, grants from Mediq Tefa, grants from NightBalance, grants from Heinen & Löwenstein, grants from AstraZen, grants from Accuramed, grants from Bekaert Deslee Academy, and grants from UCB Pharma, outside the submitted work. All other authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
ESADA Collaborators. Ulla Anttalainen and Tarja Saaresranta (Turku University Hospital, Division of Medicine, Department of Pulmonary Diseases and Sleep Research Center, Department of Pulmonary Diseases and Clinical Allergology, University of Turku, Turku, Finland); Ferran Barbè (Servei Pneumologia Hospital Arnau de Vilanova and Hospital Santa Maria, Lleida, Spain); Ozen K. Basoglu, Canan Gunduz, and Sezai Tasbakan (Dept of Chest Diseases, Ege University School of Medicine, Izmir, Turkey); Piotr Bielicki (Dept of Internal Medicine, Pneumonology and Allergology, Warsaw Medical University, Warsaw, Poland); Izolde Bouloukaki and Sophia E. Schiza (Sleep Disorders Center, University of Crete, Heraklion, Greece); Maria R. Bonsignore and Oreste Marrone (DiBiMIS, University of Palermo and CNR, Istituto per la Ricerca e l'Innovazione Biomedica, Palermo, and Italy); Pierre Escourrou, Michel Petitjean and Gabriel Roisman (Service d’Explorations Fonctionnelles Multidisciplinaires, and Unité de Médecine du Sommeil, Hôpital Antoine Béclère, Clamart, France); Ingo Fietze, Naima Laharnar, and Thomas Penzel (Centre of Sleep Medicine, Charité Universitätsmedizin Berlin, Berlin, Germany); Jan Hedner, Ding Zou, and Ludger Grote (Sleep Medicine, Sahlgrenska University Hospital and Sahlgrenska Academy, Gothenburg, Sweden); Brian D. Kent, Walter T. McNicholas and Silke Ryan (School of Medicine and Medical Science, University College Dublin, and Department of Respiratory and Sleep Medicine, St. Vincent’s Hospital Group, Dublin, Ireland); John A. Kvamme (ENT Dept., Førde Central Hospital, Førde, Norway); Patrick Lévy, Jean-Louis Pépin and Sebastien Bailly (Université Joseph Fourier and Université Grenoble Alpes, Grenoble, France); Carolina Lombardi and Gianfranco Parati (Sleep Disorders Center, Department of Cardiovascular Neural and Metabolic Sciences, IRCCS Istituto Auxologico Italiano, Milano-Bicocca University, Milan, Italy); Juan Fernando Masa and Josep M. Montserrat (Hospital Clinic I Provincial de Barcelona, Barcelona, Spain); Athanasia Pataka (Respiratory Failure Unit, G. Papanikolau Hospital, Thessaloniki, Greece); Robert Plywaczewski and Pawel Sliwinski (Institute of Tuberculosis and Lung Diseases, Warsaw, Poland); Martin Pretl (Sleep Disorders Centre, Department of Neurology, Charles University, Prague, Czech Republic); Renata Riha (Dept of Sleep Medicine, Edinburgh Royal Infirmary, Edinburgh, UK); Richard Staats (Dept. of Pneumology, University Hospital de Santa Maria, Lisbon, Portugal); Paschalis Steiropoulos (Sleep Unit, Dept of Pneumonology, Democritus University of Thrace, Alexandroupolis, Greece); Ruzena Tkacova and Pavol Joppa (Dept of Respiratory Medicine, P.J. Safarik University, Kosice, and L. Pasteur University Hospital, Kosice, Slovakia); Johan Verbraecken and E.Petiet (Multidisciplinary Sleep Disorders Centre, Antwerp University Hospital and University of Antwerp); Georgia Trakada (Pulmonary Medicine, National and Kapodistrian University of Athens, Athens, Greece); Ondrej Ludka (Department of Cardiology, University Hospital Brno and International Clinical Research Center, St. Ann´s University Hospital, Brno, Czech Republic); Schulz R(Sleep Disorders Centre, University of Giessen, Lung Centre, Giessen, Germany); Lena Lavie and Peretz Lavie (Centre for Sleep Medicine, Technion Institute of Technology, Haifa, Israel); Hein H (Sleep Disorders Center, St. Adolf Stift, Reinbeck, Germany); Varoneckas G(Institute Psychophysiology and Rehabilitation, Palanga, Lithuania) Marta Drummondand Mafalda van Zeller (Pulmonology Department Hospital São João, Medicine Faculty of Porto University, Porto, Portugal); Zoran Dogas and Tea Galic (Sleep Medicine Center, Department of Neuroscience, University of Split School of Medicine, Split, Croatia); Haralampos Gouveris (ENT department at Mainz University Hospital, Mainz, Germany).
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
arousal index
- BMI
body mass index
- CI
confidence interval
- ESADA
European Sleep Apnea Database
- ESS
Epworth Sleepiness Scale
- ODI
oxygen desaturation index
- OR
odds ratio
- OSA
obstructive sleep apnea
- PG
polygraphy
- PSG
polysomnography
- SAH
systemic arterial hypertension
REFERENCES
- 1.McNicholas WT, Bonsignore MR, Le’vy P, Ryan S. Mild obstructive sleep apnoea: clinical relevance and approaches to management. Lancet Respir Med. 2016;4(10):826–834. 10.1016/S2213-2600(16)30146-1 [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 3.Chowdhuri S, Quan SF, et al. An official American Thoracic Society Research Statement: impact of mild obstructive sleep apnea in adults. Am J Respir Crit Care Med. 2016;193(9):e37–e54. 10.1164/rccm.201602-0361ST [DOI] [PubMed] [Google Scholar]
- 4.Hou H, Zhao Y, Yu W, et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010405. 10.7189/jogh.08.010405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tkacova R, McNicholas WT, Javorsky M, et al. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J. 2014;44(4):931–941. 10.1183/09031936.00225113 [DOI] [PubMed] [Google Scholar]
- 6.Hedner J, Grote L, Bonsignore M, et al. The European Sleep Apnoea Database (ESADA): report from 22 European sleep laboratories. Eur Respir J. 2011;38(3):635–642. 10.1183/09031936.00046710 [DOI] [PubMed] [Google Scholar]
- 7.WHO Collaborating Center for Drug Statistics Methodology . Guidelines for ATC Classification and DDD Assignment. 6th ed. Oslo, Norway: World Health Organization; 2003. [Google Scholar]
- 8.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. 10.1161/01.CIR.0000154900.76284.F6 [DOI] [PubMed] [Google Scholar]
- 9.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28(12):1462–1536. [DOI] [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 11.Escourrou P, Grote L, Penzel T, et al. The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res. 2015;24(6):730–738. 10.1111/jsr.12318 [DOI] [PubMed] [Google Scholar]
- 12.Ferber R, Millman R, Coppola M, Fleetham J, Murray CF, Iber C, et al. Portable recording in the assessment of obstructive sleep apnea. ASDA standards of practice. Sleep. 1994;17(4):378–392. 10.1093/sleep/17.4.378 [DOI] [PubMed] [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson A L Jr, Quan SF; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 14.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension—an update. Hypertension. 2014;63(2):203–209. 10.1161/HYPERTENSIONAHA.113.00613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Li Y, He F, et al. Mild-to-moderate sleep apnea is associated with incident hypertension: age effect. Sleep. 2019;42(4):zsy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31(6):795–800. 10.1093/sleep/31.6.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. 10.1001/archinte.1997.00440360178019 [DOI] [PubMed] [Google Scholar]
- 19.Theorell-Haglow J, Berne C, Janson C, Lindberg E. Obstructive sleep apnoea is associated with decreased insulin sensitivity in females. Eur Respir J. 2008;31(5):1054–1060. 10.1183/09031936.00074907 [DOI] [PubMed] [Google Scholar]
- 20.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- 21.Cui R, Tanigawa T, Sakurai S, et al. Associations of sleep-disordered breathing with excessive daytime sleepiness and blood pressure in Japanese women. Hypertens Res. 2008;31(3):501–506. 10.1291/hypres.31.501 [DOI] [PubMed] [Google Scholar]
- 22.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleepdisordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614–621. 10.1161/01.CIR.0000154540.62381.CF [DOI] [PubMed] [Google Scholar]
- 23.Kapur VK, Resnick HE, Gottlieb DJ. Sleep Heart Health Study Group . Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. 2008;31(8):1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 24.Wachter R, Lüthje L, Klemmstein D, et al. Impact of obstructive sleep apnoea on diastolic function. Eur Respir J. 2013;41(2):376–383. 10.1183/09031936.00218211 [DOI] [PubMed] [Google Scholar]
- 25.Fietze I, Penzel T, Alonderis A, et al. Management of obstructive sleep apnea in Europe. Sleep Med. 2011;12(2):190–197. 10.1016/j.sleep.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 26.Bianchi MT, Goparaju B. Potential underestimation of sleep apnea severity by at-home kits: rescoring in-laboratory polysomnography without sleep staging. J Clin Sleep Med. 2017;13(4):551–555. 10.5664/jcsm.6540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell AJ, Neill AM. Home set-up polysomnography in the assessment of suspected obstructive sleep apnea. J Sleep Res. 2011;20(1 Pt 2):207–213. 10.1111/j.1365-2869.2010.00854.x [DOI] [PubMed] [Google Scholar]
- 28.Berger G, Berger R, Oksenberg A. Progression of snoring and obstructive sleep apnoea: the role of increasing weight and time. Eur Respir J. 2009;33(4):338–345. [DOI] [PubMed] [Google Scholar]
- 29.Sahlman J, Pukkila M, Seppa J, Tuomilehto H. Evolution of mild obstructive sleep apnoea after different treatments. Laryngoscope. 2007;117(6):1107–1111. 10.1097/MLG.0b013e3180514d08 [DOI] [PubMed] [Google Scholar]
- 30.Sahlman J, Miettinen K, Peuhkurinen K, et al. Activation of inflammatory cytokine system in mild obstructive sleep apnoea. J Sleep Res. 2010;19(2):341–348. 10.1111/j.1365-2869.2009.00787.x [DOI] [PubMed] [Google Scholar]
- 31.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. 10.1371/journal.pmed.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.