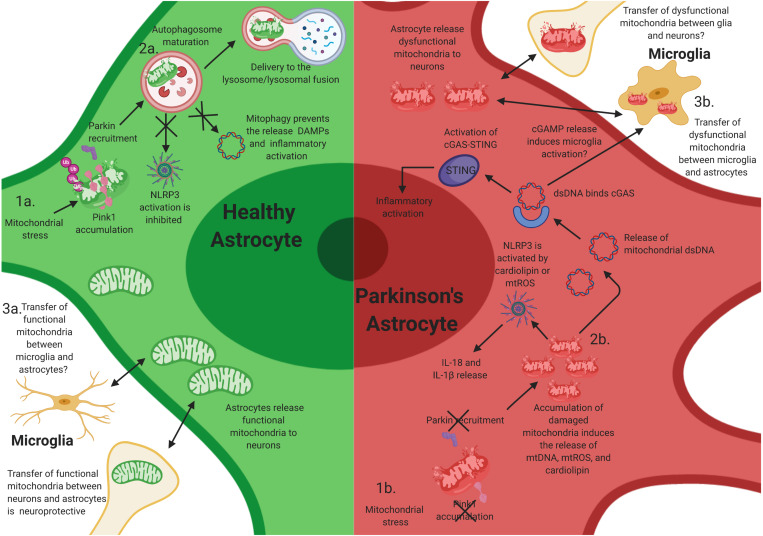
FIGURE 2.
Mitochondrial dysfunction in astrocytes contributes to neuroinflammatory activation of astrocytes. Mutations in PINK1 and Parkin likely elicit neuroinflammatory activation in astrocytes. (1a) After mitochondrial stress, PINK1 protein accumulates on the outer mitochondrial membrane. Phosphorylation of polyubiquitin and Parkin induces autophagosome maturation and eventually delivery to the lysosome. (1b) Loss-of-function (LOF) mutations in PINK1 and Parkin prevent PINK1 accumulation on the mitochondrial membrane and the activation of Parkin during mitochondrial stress, resulting in the accumulation of damaged mitochondria within astrocytes. This likely facilitates chronic inflammatory activation through multiple inflammatory pathways, including NLRP3 activation. (2a) Functional mitophagy prevents the release of damage-associated molecular patterns (DAMPs)/pathogen-associated molecular patterns (PAMPs) and activation of the NLR family, pyrin domain containing 3 (NLRP3) inflammasome in astrocytes. (2b) The accumulation of damaged mitochondria likely results in the release of intracellular DAMPs/PAMPs, cardiolipin, and mtROS, which activates NLRP3 and the cyclic guanosine monophosphate–adenosine monophosphate cGAMP synthase (cGAS)/stimulator of interferon genes (STING) pathway, resulting in inflammatory activation and release of interleukin (IL)-1β and IL-18, and potentially inducing the activation of microglia via extracellular release of cGAMP. (3a) Transfer of functional mitochondria from astrocytes to neurons is thought to be neuroprotective; however, whether microglia release functional mitochondria to astrocytes is elusive. (3b) Joshi et al. have shown that activated microglia can release dysfunctional and fragmented mitochondria to astrocytes. Astrocytes then transfer dysfunctional mitochondria to neurons, resulting in neuronal death. However, whether this occurs in vivo is unknown.