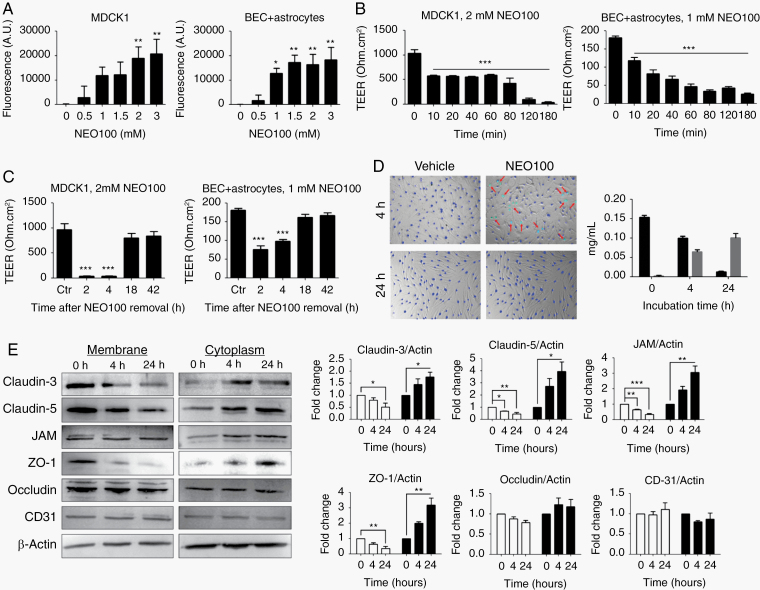
Fig. 1.
NEO100 increases cell barrier permeability. (A) The permeability of MDCK1 and patient-derived brain ECs (BEC) + astrocyte barriers at different NEO100 concentrations for 4 h was determined measuring the diffusion of an FITC-conjugated antibody across 0.4 µm pore-size Transwell inserts after 2 h at 37 ºC. *P < 0.05; **P < 0.01 (relative to vehicle-treated cells). (B) The changes of permeability with time upon treatment with NEO100 at 2 mM (MDCK1, left) or 1 mM (BEC + astrocytes, right) were studied with a TEER ohmmeter. ***P < 0.001 (relative to vehicle-treated cells). (C) After a 3-h treatment with 2 mM NEO100 (MDCK1, left) or 1 mM NEO100 (BEC + astrocytes, right), NEO100 was removed and the recovery of the membranes was determined with a TEER ohmmeter. ***P < 0.001 (relative to vehicle-treated cells). (D) BECs were treated with vehicle or 1 mM NEO100 for the indicated times. NucBlue (blue) was used to stain all cell nuclei, and NucGreen (green) was used to stain the nuclei of BECs with compromised plasma membranes (red arrows). The bar graph shows the concentration of NEO100 and its stable metabolite perillic acid remaining in the BEC supernatants after the indicated timepoints, measured by HPLC. (E) BECs were treated for 4 h or for 24 h with vehicle or 1 mM NEO100. Representative western blots showing the levels of the tight junction (TJ) or accessory proteins claudin-3, claudin-5, JAM (upper band seen in western blot), ZO-1 and occludin in the membrane or cytoplasmic fractions. CD31 was included as a control of non-TJ membrane protein. β-actin was used as a loading control. The bar graphs represent the average pixel density recorded by ImageJ of 3 independent western blots. *P < 0.05; **P < 0.01, ***P < 0.001 (relative to vehicle-treated cells).