Abstract
Background
The safety, tolerability, efficacy, and pharmacokinetics of tirabrutinib, a second-generation, highly selective oral Bruton’s tyrosine kinase inhibitor, were evaluated for relapsed/refractory primary central nervous system lymphoma (PCNSL).
Methods
Patients with relapsed/refractory PCNSL, Karnofsky performance status ≥70, and normal end-organ function received tirabrutinib 320 and 480 mg once daily (q.d.) in phase I to evaluate dose-limiting toxicity (DLT) within 28 days using a 3 + 3 dose escalation design and with 480 mg q.d. under fasted conditions in phase II.
Results
Forty-four patients were enrolled; 20, 7, and 17 received tirabrutinib at 320, 480, and 480 mg under fasted conditions, respectively. No DLTs were observed, and the maximum tolerated dose was not reached at 480 mg. Common grade ≥3 adverse events (AEs) were neutropenia (9.1%), lymphopenia, leukopenia, and erythema multiforme (6.8% each). One patient with 480 mg q.d. had grade 5 AEs (pneumocystis jirovecii pneumonia and interstitial lung disease). Independent review committee assessed overall response rate (ORR) at 64%: 60% with 5 complete responses (CR)/unconfirmed complete responses (CRu) at 320 mg, 100% with 4 CR/CRu at 480 mg, and 53% with 6 CR/CRu at 480 mg under fasted conditions. Median progression-free survival was 2.9 months: 2.1, 11.1, and 5.8 months at 320, 480, and 480 mg under fasted conditions, respectively. Median overall survival was not reached. ORR was similar among patients harboring CARD11, MYD88, and CD79B mutations, and corresponding wild types.
Conclusion
These data indicate favorable efficacy of tirabrutinib in patients with relapsed/refractory PCNSL.
Trial registration
JapicCTI-173646.
Keywords: Bruton’s tyrosine kinase, CARD11, MYD88, primary central nervous system lymphoma, tirabrutinib
Key Points.
The maximum tolerated dose of tirabrutinib was not reached at 480 mg q.d. in phase I.
Tirabrutinib showed favorable efficacy in patients with relapsed/refractory PCNSL.
Overall response rates were irrespective of mutations in CARD11, MYD88, and CD79B.
Importance of the Study.
PCNSL is a highly aggressive non-Hodgkin lymphoma with poor prognosis. Combination chemotherapy including high-dose methotrexate with whole-brain radiotherapy provides well benefits for treatment-naïve patients with PCNSL, but the disease has often relapsed. Here, we conducted a phase I/II study of tirabrutinib, a second-generation, potent, selective Bruton’s tyrosine kinase inhibitor, in relapsed or refractory PCNSL. Maximum tolerated dose was not reached at 480 mg in the 28-day phase I part. Thereafter, we observed multiple AEs, and hence tested 2 additional regimens in the phase II part: 320 mg tirabrutinib and administration of 480 mg under fasted conditions. On the basis of the results of higher rates and longer durations of response regardless of mutations in CARD11, preferred tolerability, and manageable safety profile, administration of 480 mg under fasted conditions would be the optimum regimen.
Primary central nervous system lymphoma (PCNSL) is a highly aggressive non-Hodgkin lymphoma that arises most often in the brain and sometimes affects the eyes and cerebrospinal fluid (CSF).1–3 Pathologic analysis revealed that most (~95%) PCNSLs are diffuse large B-cell lymphoma (DLBCL),4 and the DLBCL subtype of PCNSL is often classified as the non–germinal center B-cell (GCB)-like type.5 The prognosis is generally poor.6 Age over 50 years and Karnofsky performance status (KPS) <70 have consistently been identified as negative prognostic factors.7 High-dose methotrexate (HD-MTX) in combination with other chemotherapeutic agents followed by whole-brain radiotherapy (WBRT) at a dose of 30–40 Gy is used as a standard treatment for newly diagnosed patients with PCNSL in Japan. Since WBRT is associated with the risk of inducing leukoencephalopathy and cognitive impairment and decreases quality of life in patients with PCNSL, other therapeutic options have also been evaluated, including a combinatory therapy of rituximab with HD-MTX, vincristine, and procarbazine followed by a reduced dose of WBRT and then high-dose cytarabine.8
More than half of the initial responders relapse,9 and optimal treatment for recurrent patients has not been established. Re-administration of HD-MTX ± rituximab based therapy10 has been attempted for recurrent patients who responded to initial HD-MTX therapy. Monotherapies with rituximab, topotecan, and temsirolimus have also been investigated, with reported response rates of 36%,11 33%,12 and 54%,13 respectively. However, all of these monotherapies result in progression-free survival (PFS) of about 2 months and do not lead to a long-term cure. A clinical trial of lenalidomide monotherapy showed median PFS of 6 months, and those of a high dose of chemotherapy followed by autologous stem cell transplantation demonstrated prolonged median PFS of 11.6–12.4 months but high toxicity.14–16 Thus, treatment options are particularly limited for patients with relapsed/refractory PCNSL. Further, long-term treatment is undesirable, highlighting the unmet medical need.
Analysis of driver genes using whole-genome sequencing and single nucleotide polymorphism arrays revealed that the frequencies of MYD88 and CD79B mutations, which affect the B-cell receptor (BCR) of antigen signaling activation, were higher in PCNSL biopsy samples compared with conventionally reported activated B-cell-DLBCL.17 Since Bruton’s tyrosine kinase (BTK) is a mediator located downstream of BCR and is associated with various B-cell malignancies,18 BTK inhibitors may be effective for PCNSL. Recently, multiple studies have evaluated the first-generation BTK inhibitor ibrutinib in relapsed/refractory PCNSL. More than half of patients with PCNSL responded to ibrutinib in the salvage setting.19–22 Particularly, a subset of patients showed complete responses (CR). Interestingly, efficacy of ibrutinib seemed independent of the MYD88 and CD79B mutations. Thus, treatment using BTK inhibitors is still investigational, but has been promising.
In contrast to ibrutinib that potently inhibits multiple off-target kinases, which may lead to serious toxicities,23–27 tirabrutinib (ONO/GS-4059, Ono Pharmaceutical)28 is a second-generation, potent, highly selective, irreversible oral BTK inhibitor.29 A European phase I study assessed the tolerability and safety in relapsed/refractory B-cell non-Hodgkin lymphoma (B-NHL) patients and chronic lymphocyte leukemia (CLL) patients. The maximum tolerated dose (MTD) was 480 mg once daily (q.d.) and 600 mg q.d. for B-NHL and CLL patients, respectively.25 A Japanese phase I study in patients with relapsed/refractory B-NHL and CLL30 is currently under way to investigate the safety and efficacy of tirabrutinib. No dose-limiting toxicities (DLTs) were observed in 2 or more patients in any cohort, and the tolerability of tirabrutinib was confirmed at a q.d. dose of 480 mg and the twice-daily dose of 300 mg in Japanese patients. This phase I/II study aimed to confirm the safety, tolerability, efficacy, and pharmacokinetics of tirabrutinib and explore the genetic features of Japanese patients with relapsed/refractory PCNSL.
Materials and Methods
Study Design and Treatment
This was a multicenter, open-label, uncontrolled phase I/II study conducted in Japan at 10 sites between October 2017 and June 2019. The study was registered at Clinicaltrials.jp under the identifier JapicCTI-173646 (https://database.japic.or.jp). Ethical approval was received by the institutional review boards of participating study sites, and all patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
The study consisted of 2 parts: phase I and phase II. Phase I had an open-label, dose-escalation design and aimed to confirm the tolerability of tirabrutinib in patients with relapsed or refractory PCNSL, to determine the doses for phase II, and to investigate the pharmacokinetics of tirabrutinib. Phase II had an open-label, uncontrolled design and aimed to investigate efficacy with the primary endpoint of overall response rate (ORR; central assessment) at best response. Patients with relapsed/refractory PCNSL were also assessed for safety at the doses of tirabrutinib determined in phase I.
In phase I, tirabrutinib was orally administered at a dose of 320 mg q.d. in cohort 1 and 480 mg q.d. in cohort 2 for one 28-day cycle (DLT evaluation period). Of the patients who were enrolled in cohort 1, the dose could be increased to the cohort 2 dose in patients who continued to receive tirabrutinib when the tolerability of cohort 2 was confirmed. Patients started treatment in cohort 1 and transitioned to cohort 2 according to the transition rules (see Supplementary Text and Supplementary Table 1). Maximum dose was set to 480 mg q.d. on the basis of the results of the MTD in the previous dose-escalation studies of tirabrutinib.25,30 Treatment was continued until disease progression or clinically unacceptable toxicity was observed. After the DLT evaluation period (cycle 1) ended, patients could transition to the extension period at the same dose if they did not present DLTs or the investigator deemed the patient capable of transitioning to the extension period. In phase II, tirabrutinib was orally administered q.d. at the dose determined in phase I for one 28-day cycle. If the cohort 1 dose was the MTD, the cohort 1 dose was used as the dose for phase II.
Treatment was interrupted in patients with grade 4 thrombocytopenia or grade 3 thrombocytopenia with bleeding, grade 4 neutropenia or febrile neutropenia, grade 2 or 3 pneumonitis, or grade ≥3 non-hematological toxicity other than pneumonitis and skin-related disorder. In case these events recovered to one grade or resolved, tirabrutinib treatment could be resumed at the same dose before the interruption. If toxicity recurred, the dose could be lowered by one step. The method used to reduce the tirabrutinib dose is shown in Supplementary Table 2; the lowest allowed dose was 160 mg q.d. Prohibited therapies are described in the Supplementary Text.
Patients
Inclusion criteria included men and women aged ≥20 years, histopathologically diagnosed as PCNSL without systemic tumor, who had relapsed or refractory PCNSL, had received at least one prior therapy, with a cerebral lesion >1.0 cm in the longest diameter on gadolinium-enhanced nuclear MRI within 14 days prior to the first tirabrutinib dose, KPS of 70–100, expected survival ≥3 months, able to comply with the study procedures and testing, receiving appropriate contraception methods if of reproductive age, and met the following laboratory criteria: neutrophil count ≥1000/mm3, platelet count ≥50 000/mm3, hemoglobin ≥8.0 g/dL, aspartate aminotransferase and alanine aminotransferase <2.5 times the upper limit of normal (ULN), total bilirubin <1.5 times ULN, and creatinine clearance ≥50 mL/min (Cockcroft–Gault formula) or ≥50 mL/min/1.73 m2 (Modification of Diet in Renal Disease formula).
The main exclusion criteria are provided in the Supplementary Text. Briefly, excluded were patients with PCNSL without a B-cell neoplasm, who recently received chemotherapy or radiotherapy or who had severe disease complications, severe comorbidities, or infectious diseases, including human immunodeficiency virus and cytomegalovirus. Patients who continuously received systemic corticosteroid within 14 days prior to the first dose of tirabrutinib were also excluded; up to 10 mg/day and 25 mg/day of prednisolone or equivalent for any symptoms other than PCNSL and for symptoms of PCNSL such as edema, respectively, were permitted.
Study Endpoints
Safety
.—Adverse events (AEs) and laboratory (hematology, blood biochemistry, and urinalysis) abnormalities were assessed. AEs were assessed according to the National Cancer Institute–Common Terminology Criteria for Adverse Events v4.0 Japan Clinical Oncology Group version.
Efficacy
.—The primary efficacy endpoint was ORR at best response (CR, unconfirmed complete response [CRu], partial response [PR]) assessed by a central independent review committee. Complete response (CR + CRu) was also assessed. Tumor response was assessed, according to the International PCNSL Collaborative Group criteria, by MRI before the first dose of tirabrutinib, at day 28 of cycle 1, and at day 1 of each odd-numbered cycle. Secondary efficacy endpoints were best overall response, duration of response, disease-free survival, time-to-treatment response, PFS, and overall survival (OS).
Genetic testing.
—DNA was extracted from formalin-fixed, paraffin-embedded tumor samples of the brain biopsies for determination of the MYD88, CD79A, CD79B, and CARD11 somatic mutations. Additional details of methods used for genetic testing are provided in the Supplementary Text and Supplementary Table 3.
Pharmacokinetics
.—Calculated pharmacokinetic parameters were maximum observed plasma concentration, area under the plasma concentration-time curve, and trough concentration of tirabrutinib in CSF. Details of sampling are provided in the Supplementary Text.
Statistical Analysis
The sample size rationale and definitions of the study analysis sets (safety analysis set, full analysis set, DLT population, and pharmacokinetics analysis set) are provided in the Supplementary Text. The number of patients required was 15 or 20 to secure a two-tailed significance level of 5.0% and power of 80% or 90%, and to account for possible dropouts.
ORR (primary efficacy analysis) was calculated by dosage regimen at registration and overall (central assessment). In addition, the two-tailed 95% confidence interval (CI) for ORR was calculated using the Clopper–Pearson method. For secondary efficacy analysis, percentages were calculated by dosage regimen at registration and overall for complete remission rate and best overall response. In addition, their two-tailed 95% CIs were calculated using the Clopper–Pearson method. Waterfall plots were generated by dosage regimen at registration for the maximum percent change in the sum of the products of the diameters of target lesions. The software used for the statistical analysis was SAS v9.4.
Results
Patients
Between October 19, 2017 and June 13, 2019, forty-four patients were enrolled (Supplementary Figure 1). Overall, 20 patients were treated with tirabrutinib 320 mg and 24 patients with tirabrutinib 480 mg, including 17 patients under fasted conditions. The median follow-up period was 9.1 months (range, 1.4–19.4) in the whole group: 11.3 months, 15.6 months, and 3.8 months in the 320 mg q.d., 480 mg q.d., and 480 mg q.d. fasted groups, respectively. A total of 27 patients discontinued the treatment; 21 for PD, 3 for AEs, and 3 for other reasons.
The median patient age was 60 years (range, 29–86) and KPS was 80 (range, 70–100) (Table 1). The median number of prior therapies for PCNSL was 2 (range, 1–14); all patients had received HD-MTX based chemotherapy; and 32 (72.7%) had isolated intracerebral lesions, 9 (20.5%) had CSF involvement, and 3 (6.8%) had intraocular involvement. Compared with the other groups, the median KPS was the lowest; 7 (41.2%) of 17 patients had CSF involvement; and the median tumor burden was higher and with multiple oncogenic mutations in the 480 mg fasted group. Among 44 patients, prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMZ), pentamidine isethionate, and ganciclovir was received by 36 (81.8%), 9 (20.5%), and 1 (2.3%), respectively. At the first dose of tirabrutinib, 13 (29.5%) patients received corticosteroids at daily doses of 3–25 mg prednisolone to control symptoms of PCNSL, including edema. Of those, 9 patients responded, and 7 of the responders were still on corticosteroids at the time of their best responses.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | 320 mg q.d. N = 20 | 480 mg q.d. N = 7 | 480 mg q.d., fasted N = 17 | All N = 44 |
|---|---|---|---|---|
| Sex, male, n (%) | 14 (70.0) | 4 (57.1) | 6 (35.3) | 24 (54.5) |
| Age, median, y (range) | 59.5 (41–86) | 54.0 (42–75) | 65.0 (29–85) | 60.0 (29–86) |
| Karnofsky performance status, n (%) | ||||
| <70 | 0 | 0 | 0 | 0 |
| 70 | 8 (40.0) | 3 (42.9) | 9 (52.9) | 20 (45.5) |
| 80 | 2 (10.0) | 0 (0.0) | 2 (11.8) | 4 (9.1) |
| 90 | 6 (30.0) | 2 (28.6) | 3 (17.6) | 11 (25.0) |
| 100 | 4 (20.0) | 2 (28.6) | 3 (17.6) | 9 (20.5) |
| Median (range) | 85.0 (70–100) | 90.0 (70–100) | 70.0 (70–100) | 80.0 (70–100) |
| Number of previous lines of treatment, n (%) | ||||
| 1 | 9 (45.0) | 2 (28.6) | 7 (41.2) | 18 (40.9) |
| 2–3 | 5 (25.0) | 3 (42.9) | 8 (47.1) | 16 (36.4) |
| ≥4 | 6 (30.0) | 2 (28.6) | 2 (11.8) | 10 (22.7) |
| Median (range) | 2.0 (1–6) | 2.0 (1–14) | 2.0 (1–5) | 2.0 (1–14) |
| Previous methotrexate | 20 (100.0) | 7 (100.0) | 17 (100.0) | 44 (100.0) |
| Previous rituximab | 13 (65.0) | 3 (42.9) | 10 (58.8) | 26 (59.1) |
| Previous whole-brain radiotherapy | 14 (70.0) | 5 (71.4) | 10 (58.8) | 29 (65.9) |
| Previous HCT-ASCT | 2 (10.0) | 1 (14.3) | 4 (37.8) | 7 (15.9) |
| Disease status, n (%) | ||||
| Relapsea | 17 (85.0) | 2 (28.6) | 14 (82.4) | 33 (75.0) |
| Refractoryb | 3 (15.0) | 3 (42.9) | 3 (17.6) | 9 (20.5) |
| Unknown | 0 | 2 (28.6) | 0 | 2 (4.5) |
| CNS involvement, n (%) | ||||
| CSF | ||||
| Positive | 1 (5.0) | 1 (14.3) | 7 (41.2) | 9 (20.5) |
| Negative | 19 (95.0) | 6 (85.7) | 10 (58.8) | 35 (79.5) |
| IOL | ||||
| Positive | 2 (10.0) | 0 | 1 (5.9) | 3 (6.8) |
| Minor RPE abnormality | 5 (25.0) | 0 | 1 (5.9) | 6 (13.6) |
| Negative | 13 (65.0) | 7 (100.0) | 15 (88.2) | 35 (79.5) |
| GCB subtype, n (%) | ||||
| GCB | 7 (35.0) | 1 (14.3) | 5 (29.4) | 13 (29.5) |
| Non-GCB | 13 (65.0) | 6 (85.7) | 12 (70.6) | 31 (70.5) |
| Oncogenic mutation, n (%) | ||||
| CARD11 | 3 (15.0) | 2 (28.6) | 12 (70.6) | 17 (38.6) |
| MYD88 | 15 (75.0) | 6 (85.7) | 11 (64.7) | 32 (72.7) |
| CD79B | 10 (50.0) | 0 | 8 (47.1) | 18 (40.9) |
| Sum of the products of the greatest diameters at target lesion, mm2, n (%) | ||||
| <400 | 13 (65.0) | 3 (42.9) | 7 (41.2) | 23 (52.3) |
| ≥400 | 7 (35.0) | 4 (57.1) | 10 (58.8) | 21 (47.7) |
| Median, mm2 (range) | 233.66 (56.3–2031.4) | 514.99 (77.1– 4020.5) | 618.58 (86.5–2047.7) | 385.84 (56.3–4020.5) |
Abbreviations: HCT-ASCT, high-dose chemotherapy followed by autologous stem cell transplantation; IOL, intraocular lymphoma; RPE, retinal pigment epithelium.
aA disease that responded to the last therapy (CR, CRu, or PR) but progressed afterward was defined as relapsed PCNSL.
bA disease that did not respond to the last therapy (stable or progressive disease) was defined as refractory PCNSL.
In phase I, no DLTs were observed and the MTD was not reached at 480 mg, so the recommended dose for phase II was determined as 480 mg. After the DLT evaluation, 1 patient who received a 480-mg tirabrutinib died of pneumocystis jirovecii pneumonia and interstitial lung disease (ILD) that occurred at the same timing. This patient had grade 3 lymphopenia and received 2 mg of betamethasone for the control of brain edema before receiving the treatment with the study drug but did not have prophylactic therapy with TMP-SMZ. In addition, in phase II, 3 of 4 patients treated with 480 mg had grade 3 skin-related disorders (2 events of erythema multiforme and 1 event of drug eruption). Therefore, the dose for phase II was reduced from 480 mg to 320 mg; however, the treatment response and duration were lower than with the 480 mg dose. Two clinical studies have shown that treatment under fasted condition was well tolerable and safe with a dose of 480 mg tirabrutinib.25,30 Thus, 17 patients were additionally recruited and received a dose of 480 mg in a fasted state, instead of increasing the dose for patients treated with the 320 mg dose.
Study Endpoints
Safety
.—Commonly observed AEs of any grade were rash (31.8%), neutropenia (22.7%), leukopenia (18.2%), and lymphopenia (15.9%) (Table 2). Commonly observed grade ≥3 AEs were neutropenia (9.1%), lymphopenia, leukopenia, and erythema multiforme (6.8% each). One patient had grade 5 AEs (pneumocystis jirovecii pneumonia and ILD) 32 days after starting tirabrutinib 480 mg q.d.
Table 2.
Adverse events of any grade with a frequency >10%
| AEs, n (%) | 320 mg q.d. N = 20 | 480 mg q.d. N = 7 | 480 mg q.d., Fasted N = 17 | All N = 44 | ||||
|---|---|---|---|---|---|---|---|---|
| All Grades | Grade ≥3 | All Grades | Grade ≥3 | All Grades | Grade ≥3 | All Grades | Grade ≥3 | |
| Any AEs | 16 (80.0) | 6 (30.0) | 7 (100.0) | 5 (71.4) | 15 (88.2) | 10 (58.8) | 38 (86.4) | 21 (47.7) |
| Rash | 6 (30.0) | 0 | 2 (28.6) | 0 | 6 (35.3) | 1 (5.9) | 14 (31.8) | 1 (2.3) |
| Neutropenia | 4 (20.0) | 2 (100) | 2 (71.4) | 0 | 4 (20.0) | 2 (11.8) | 10 (22.7) | 4 (9.1) |
| Leukopenia | 2 (10.0) | 1 (5.0) | 3 (42.9) | 0 | 3 (17.6) | 2 (11.8) | 8 (18.2) | 3 (6.8) |
| Lymphopenia | 2 (10.0) | 0 | 3 (42.9) | 2 (28.6) | 2 (11.8) | 1 (5.9) | 7 (15.9) | 3 (6.8) |
| Thrombocytopenia | 1 (5.0) | 0 | 3 (42.9) | 0 | 1 (5.9) | 0 | 5 (11.4) | 0 |
| Erythema multiforme | 2 (10.0) | 1 (5.0) | 3 (42.9) | 2 (28.6) | 0 | 0 | 5 (11.4) | 3 (6.8) |
| Constipation | 1 (5.0) | 0 | 0 | 0 | 4 (23.5) | 0 | 5 (11.4) | 0 |
| Drug eruption | 2 (10.0) | 1 (5.0) | 1 (14.3) | 1 (14.3) | 1 (5.9) | 0 | 4 (9.1) | 2 (4.5) |
| Blood bilirubin increased | 4 (20.0) | 1 (5.0) | 0 | 0 | 0 | 0 | 4 (9.1) | 1 (2.3) |
| Nausea | 1 (5.0) | 0 | 1 (14.3) | 0 | 2 (11.8) | 1 (5.9) | 4 (9.1) | 1 (2.3) |
| Stomatitis | 2 (10.0) | 0 | 1 (14.3) | 0 | 1 (5.9) | 0 | 4 (9.1) | 0 |
| Anemia | 1 (5.0) | 0 | 1 (14.3) | 0 | 2 (11.8) | 0 | 4 (9.1) | 0 |
| Vomiting | 1 (5.0) | 0 | 0 | 0 | 3 (17.6) | 0 | 4 (9.1) | 0 |
| Aspartate aminotransferase increased | 2 (10.0) | 1 (5.0) | 0 | 0 | 1 (5.9) | 1 (5.9) | 3 (6.8) | 2 (4.5) |
| Hypertriglyceridemia | 0 | 0 | 0 | 0 | 3 (17.6) | 2 (11.8) | 3 (6.8) | 2 (4.5) |
| Dyslipidemia | 1 (5.0) | 0 | 1 (14.3) | 0 | 1 (5.9) | 1 (5.9) | 3 (6.8) | 1 (2.3) |
| Seizure | 1 (5.0) | 0 | 0 | 0 | 2 (11.8) | 1 (5.9) | 3 (6.8) | 1 (2.3) |
| Rash maculopapular | 1 (5.0) | 0 | 0 | 0 | 2 (11.8) | 1 (5.9) | 3 (6.8) | 1 (2.3) |
| Urinary tract infection | 0 | 0 | 2 (28.6) | 0 | 1 (5.9) | 1 (5.9) | 3 (6.8) | 1 (2.3) |
| Headache | 2 (10.0) | 0 | 1 (14.3) | 0 | 0 | 0 | 3 (6.8) | 0 |
In total, 13 serious AEs in 9 patients were reported, including 8 adverse drug reactions in 6 patients (erythema multiforme, n = 2; two patients), pneumocystis jirovecii pneumonia and ILD (each n = 1; both events occurred simultaneously in the same patient), drug eruption and pneumonia (n = 1 each; one patient each), hematuria and bronchopulmonary aspergillosis (each n = 1; both events occurred in the same patient). Three patients discontinued tirabrutinib due to AEs: grade 3 drug eruption (n = 2; one patient each in 320 mg and 480 mg groups) and pneumocystis jirovecii pneumonia and ILD (each n = 1; both events occurred in the same patient). Only 1 death due to AEs occurred during the study as described above.
Across treatment groups, the overall incidence rate of skin-related disorders was 54.5% (24/44): rash (31.8%), erythema multiforme (11.4%), drug eruption (9.1%), rash maculopapular (6.8%), and blister (2.3%). Of these, 6.8% of erythema multiforme, 4.5% of drug eruption, and 2.3% of rash and rash maculopapular events were grade ≥3 treatment-emergent AEs. Five (71.4%) of the 7 patients who developed grade ≥3 skin-related disorders had TMP-SMZ as a concomitant drug for pneumonia prophylaxis. All events recovered after treatment interruption or treatment with antihistamine and topical steroids, and patients were able to continue the treatment, except for 2 patients with drug eruption. The overall incidence rate of infection was 31.8% (14/44) with 4 grade ≥3 AEs. No cardiovascular-related AEs were observed, such as atrial fibrillation and hypotension, at any grade.
Efficacy
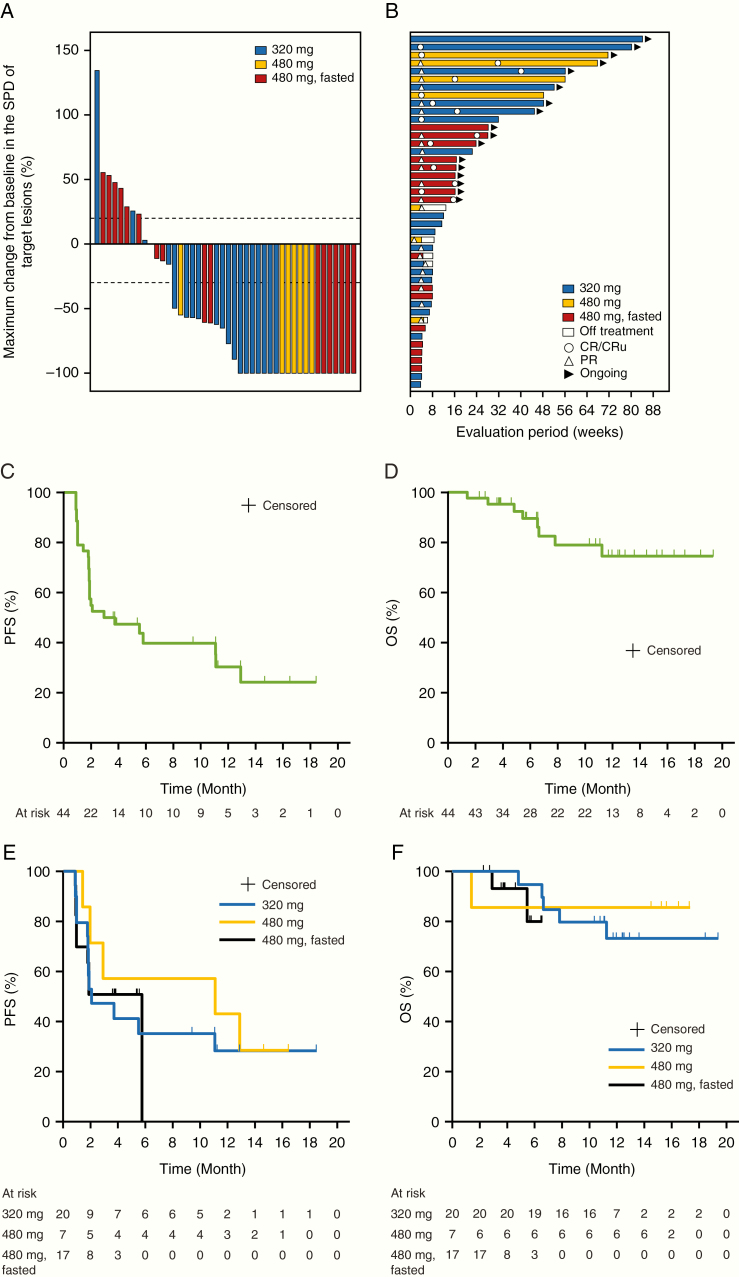
.—Overall, significant clinical responses were observed at each dose level (Fig. 1A, B) and the central independent review committee–assessed ORR was 63.6% (28/44). ORR was 60.0% (12/20 patients) with 5 CR/CRu at 320 mg, 100.0% (7/7 patients) with 4 CR/CRu at 480 mg, and 52.9% (9/17 patients) with 6 CR/CRu at 480 mg under fasted conditions (Table 3). The best overall responses in 11 patients were evaluated as CRu because of observation of minimal disease brain imaging (3 patients), minimal retinal pigment epithelium abnormality (2 patients), continuous administration of corticosteroid (3 patients), and no confirmation test performed (3 patients). All patients with concurrent intraocular lymphoma achieved PR (3/3 patients). Among 9 patients with CSF positivity, 5 (55.6%) achieved PR or more, with 3 achieving CSF negativity after the treatment.
Fig. 1.
(A) Maximum change in the SPD, (B) swimmer plot of overall response rate, (C) PFS and (D) OS in all patients, and (E) PFS and (F) OS by tirabrutinib dose during the study. SPD, sum of the product of the greatest diameters.
Table 3.
Best overall response and PFS
| 320 mg q.d. N = 20 | 480 mg q.d. N = 7 | 480 mg q.d., Fasted N = 17 | All N = 44 | |
|---|---|---|---|---|
| Overall response rate (CR + CRu + PR) | 12 | 7 | 9 | 28 |
| n % (95% CI) | 60.0 (36.1–80.9) | 100.0 (59.0–100.0) | 52.9 (27.8–77.0) | 63.6 (47.8–77.6) |
| Complete response rate (CR + CRu) | 5 | 4 | 6 | 15 |
| n % (95% CI) | 25.0 (8.7–49.1) | 57.1 (18.4–90.1) | 35.3 (14.2–61.7) | 34.1 (20.5–49.9) |
| Best overall response, n (%) | ||||
| CR | 2 (10.0) | 1 (14.3) | 1 (5.9) | 4 (9.1) |
| CRu | 3 (15.0) | 3 (42.9) | 5 (29.4) | 11 (25.0) |
| PR | 7 (35.0) | 3 (42.9) | 3 (17.6) | 13 (29.5) |
| SD | 4 (20.0) | 0 | 3 (17.6) | 7 (15.9) |
| PD | 4 (20.0) | 0 | 5 (29.4) | 9 (20.5) |
| NE | 0 | 0 | 0 | 0 |
| Median PFS, mo (95% CI) | 2.1 (1.8–NE) | 11.1 (1.4–NE) | 5.8 (1.0–5.8) | 2.9 (1.8–11.1) |
Abbreviations: SD, stable disease; PD, progressive disease; NE, not evaluable.
Overall, the median PFS was 2.9 months (95% CI: 1.8–11.1) (Fig. 1C). The median PFS was 2.1 months (95% CI: 1.8–not evaluable [NE]) at 320 mg, 11.1 months (95% CI: 1.4–NE) at 480 mg, and 5.8 months (95% CI: 1.0–5.8) at 480 mg under fasted conditions. Median OS was not reached (95% CI: NE–NE) (Fig. 1D). Four patients who relapsed after HD-MTX without WBRT pretreatment had been treated with tirabrutinib for more than 12 months without remission of the tumor.
All patients harbored at least one of the MYD88, CD79B, and CARD11 mutations, including non-oncogenic gain-of-function mutations. There were no marked differences in the ORR between patients harboring each oncogenic mutant and the corresponding wild type; CARD11 (58.8% [10/17] vs 66.7% [18/27]), MYD88 (59.4% [19/32] vs 75.0% [9/12]), CD79B (50.0% [9/18] vs 73.1% [19/26]), respectively. Of 9 patients, including 5 in the 320 mg group and 4 at the 480 mg group, with durable response for 6 months or more, 5 (55.6%) patients harbored mutations in CARD11, 7 (77.8%) in MYD88, and 3 (33.3%) in CD79B, indicating no remarkable difference in proportions of these mutations between durable responders and the whole population.
Non-GCB type assessed by Hans criteria was 70.5%. A total of 72.7% of the evaluated PCNSLs exhibited oncogenic mutations in MYD88 (L265P); 40.9% had constitutively active mutations of CD79B; and 38.6% had oncogenic mutations of CARD11, which is higher than the previously reported rates of CARD11 mutation in PCNSL.31,32 These data indicate that the canonical CARD11 mutation is more frequent, as well as MYD88 and CD79B in PCNSLs, than in previously reported non-GCB DLBCLs (MYD88, 29%; CD79B, 18%; CARD11, 10%).33 In our study, mutations in the BCR–nuclear factor-kappaB pathways were also identified in both germinal center and non–germinal center tumors (Table 4 and Supplementary Table 4).
Table 4.
Relationship between mutations and clinical response
| Usage/Dose | Best Response | GCB Subtype | CARD11 | CD79A | CD79B | MYD88 | ORR‡ |
|---|---|---|---|---|---|---|---|
| 320 mg | PD | NGC | F115I | WT | WT | WT | 57% (4/7) |
| 320 mg | PR | GCB | D200N | WT | WT | WT | |
| 480 mg, fasted | PD | GCB | D224N | WT | WT | WT | |
| 480 mg, fasted | SD | NGC | M275I | WT | WT | WT | |
| 480 mg, fasted | PR | NGC | N255D | WT | WT | WT | |
| 480 mg, fasted | PR | NGC | E185A | WT | WT | WT | |
| 480 mg | PR | NGC | F130L | WT | WT | WT | |
| 480 mg, fasted | PD | NGC | E185A | WT | Y196H | L265P | 33% (2/6) |
| 480 mg, fasted | PD | NGC | E185A | WT | A188G/E224D | L265P | |
| 480 mg, fasted | PD | GCB | Q48* | G167S/L168F | Y196C/D202N | L265P/K269E | |
| 480 mg, fasted | SD | NGC | E185A | WT | Y196S | L265P | |
| 480 mg, fasted | CRu | NGC | C49Y | WT | E229K | Q262* | |
| 480 mg, fasted | CRu | GCB | E185A | WT | Y196H | L265P |
CRu, unconfirmed complete response; GCB, germinal center-B-cell; NGC, non-germinal center subtype; PR, partial response; SD, stable disease; PD, progressive disease; WT, wild type.
‡ORR was defined as the percentage of patients (number of patients with response/treated).
Pharmacokinetics
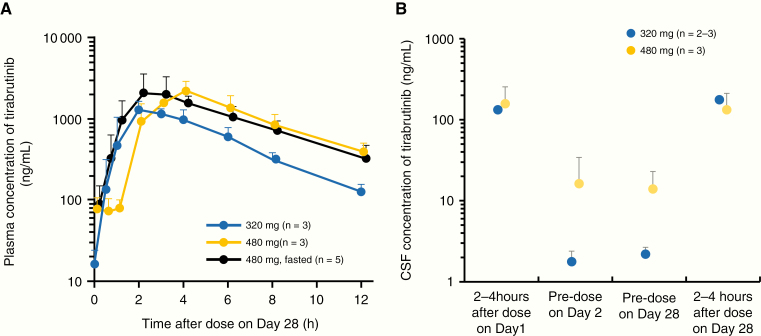
.—Intensive pharmacokinetic samplings were conducted from 3 patients in the 320 mg group, 3 patients in the 480 mg group, and 6 patients in the 480 mg under fasted conditions group. Fig. 2A, B shows the plasma and CSF concentrations of tirabrutinib. Mean values with standard deviations of maximum observed plasma concentration of tirabrutinib after multiple administration on day 28 at 320 mg, 480 mg, and 480 mg under fasted conditions were 1360 ± 229, 2270 ± 556, and 2690 ± 1120 ng/mL, respectively. The area under the plasma concentration-time curve over a dosing interval was 6870 ± 898, 13500 ± 3260, and 13400 ± 3910 ng·h/mL, respectively. Trough concentrations of tirabrutinib in CSF/plasma at 320 mg and 480 mg on day 28 were (2.19 ± 0.476)/(16.3 ± 7.71) ng/mL and (14.0 ± 8.92)/(77.0 ± 28.5) ng/mL, respectively.
Fig. 2.
(A) Mean plasma concentration of tirabrutinib on day 28 of cycle 1. Pharmacokinetic data on day 28 in 1 out of 6 patients at the 480 mg fasted group in the pharmacokinetic analysis were unavailable. Error bars indicate standard deviation. (B) Mean CSF concentration of tirabrutinib. Data in 1 patient on day 1 and 2–4 hours after dose on day 2 were unavailable. Error bars indicate standard deviation.
Discussion
Given the lack of standard treatment for relapsed/refractory PCNSL; the recent spike in the incidence of PCNSL, especially in elderly populations; and the limited treatment alternatives available to PCNSL patients, particularly those with relapsed/refractory disease, this study aimed to evaluate the safety, tolerability, efficacy, and pharmacokinetics of tirabrutinib monotherapy for such patients in Japan. It is noteworthy that no DLTs were observed in phase I of this study, and the MTD was not reached at 480 mg. However, in the 480 mg dose group, one patient presented with pneumocystis jirovecii pneumonia and ILD at the same timing, and in phase II, 3 of 4 patients treated with 480 mg had grade 3 skin-related disorders (2 events of erythema multiforme and 1 event of drug eruption); thus, the dose for phase II was reduced from 480 mg to 320 mg. Given the marked difference in efficacy with the 480 mg dose, the 480 mg dose was reinstated in 17 patients in a fasted state.
Regarding the safety profile, nearly half of patients had a grade ≥3 AE (47.7%), of which neutropenia, lymphopenia, leukopenia, and erythema multiforme were the most frequent. However, only 6 patients presented serious adverse drug reactions: erythema multiforme (2 patients); drug eruption and pneumonia (1 patient each); hematuria and bronchopulmonary aspergillosis (both in the same patient); and ILD and pneumocystis jirovecii pneumonia (both in the same patient). Three of these serious AEs (2 events of drug eruption and 1 case of ILD and pneumocystis jirovecii pneumonia) led to discontinuation of the study treatment in these patients. Notably, the incidence rate of skin-related disorders was 54.5% (24/44 patients), which is higher than that observed in previous tirabrutinib studies.25,30 Although the numbers were small, the incidence rates of skin-related disorders were numerically similar with either of the doses tested. Among the 24 patients who experienced skin-related disorders, 22 were able to continue tirabrutinib treatment while receiving antihistamine drugs (n = 16), steroids (n = 17), or no concomitant medication (n = 3). Thus, skin-related disorders were manageable with appropriate management without the need to discontinue the tirabrutinib treatment.
In this study, 36 patients (81.8%) received TMP-SMZ as a prophylactic treatment for pneumonia because pneumocystis jirovecii pneumonia had been observed in patients treated with ibrutinib and the risk for opportunistic infection may be increased due to perturbation of the B-cell function by tirabrutinib. Five of 7 patients (71.4%) who developed grade ≥3 skin-related disorders had TMP-SMZ for pneumonia. Thus, the relatively higher incidence of grade ≥3 skin-related disorders in this study could be attributed to concomitant administration of TMP-SMZ and tirabrutinib. One patient who had pneumonia by pneumocystis jirovecii and ILD did not take any countermeasures against infection, which ultimately led to death according to the autopsy findings. The autopsy result indicated that the infection could have possibly caused the onset of ILD. No deaths were observed among patients who received TMP-SMZ, and therefore, prophylactic use of TMP-SMZ is recommended in the treatment with tirabrutinib.
Regarding efficacy, our results confirmed a high response rate with each tirabrutinib dose in all groups. A longer PFS and higher CR rate were observed in the 480 mg group, which had a higher trough concentration in CSF. This finding is consistent with a preclinical study.34 However, since the number of patients in the 480 mg group was small, PFS data with longer follow-up and further studies with larger cohorts are warranted.
The exposure of tirabrutinib increased with increasing dose, regardless of fasting status. According to the pharmacokinetics study in healthy subjects, food intake was associated with a slight increase in exposure of tirabrutinib. However, such an effect of food on exposure was not observed in this study. Although no statistical analysis to characterize the patient populations with 3 different treatments was planned in this study, lower ORR in the 480 mg fasted group compared with the 320 mg group may arise from the differences in patient characteristics, including lower median KPS (70 vs 85) and larger median target lesion in the sum of the product of the greatest diameters (619 mm2 vs 234 mm2).
The CSF concentration increased with increasing plasma concentration at trough, while the trough concentration ratios between CSF and plasma at each dose were comparable with the free fraction of tirabrutinib in plasma. The CSF/plasma concentration ratio of tirabrutinib was approximately 13–18%, which is higher than the published ratio of ibrutinib (1–7%).35
The efficacy and pharmacokinetics discussed above suggest that a dose of 480 mg tirabrutinib expects better effects than 320 mg, which would account for the observed difference in median PFS (11.1 or 5.78 vs 2.1 mo). Preferred tolerability and manageable safety profile were observed with a dose of up to 480 mg tirabrutinib under fasted conditions in previous tirabrutinib studies25,30 and in this study. These results suggest that 480 mg under fasted condition might be the optimum dosage regimen for patients with relapsed or refractory PCNSL.
We also observed that MYD88, CD79B, and CARD11 mutations occurred in 39–73% of PCNSL cases. Interestingly, it has been reported that both CD79B and MYD88 (L265P) mutations were associated with improved ORR for BTK inhibitors,19,20 whereas mutations in either gene were not associated with improved ORR in our study. CARD11 mutations occurred frequently in patients with PCNSL and were associated with worse prognosis based on prior studies of ibrutinib.19,36 In contrast, CARD11 mutations did not appear to affect ORR in this study, even though the proportion of CARD11 was higher than the prior studies. This discrepancy may be attributed to the difference of mutation points in CARD11 in this study and the prior study.19 However, most missense mutations identified in this study were found within the functional coiled-coil domain of CARD11 and were also reported as oncogenic mutations.37–39 In order to clarify the effect of CARD11 mutations on response, further researches may be required, such as a basic analysis of inhibitory effects of BTK inhibitors on each CARD11 mutant. Future clinical studies with larger cohorts are also warranted to evaluate relationships between oncogenic key mutations and response.
We succeeded in identifying response in patients with CARD11 mutations and found a low response rate in patients with triple mutants CD79A/B, MYD88 (L265P), and CARD11 among PCNSL patients. In the 480 mg fasted group, 3 in 5 patients with PD as best response had triple mutants, which may explain the lower ORR in this group compared with the 320 mg group. Since the relationships between mutational status and responses to BTK inhibitors involving tirabrutinib and ibrutinib have been controversial partly due to the small evaluation cohorts, further studies with larger cohorts are warranted. Understanding of the genetic abnormalities of the patients may confer advantages, particularly in the context of combination therapies and in patients intolerant to standard of care in the future.
A possible combinatory therapy of tirabrutinib plus HD-MTX or replacement of WBRT with tirabrutinib in the current standard regimen may lead to prolonged PFS and OS, which are interesting clinical questions to be evaluated in future trials.
Limitations
The main limitations of the study were the small sample size, the open-label design, the lack of randomization to treatment, and the lack of a comparator.
Conclusions
Tirabrutinib was tolerable and the MTD was not reached in patients with PCNSL. These data indicate safety and efficacy of tirabrutinib in patients with relapsed/refractory PCNSL.
Funding
This work was supported by Ono Pharmaceutical Co, Ltd, Osaka, Japan.
Supplementary Material
Acknowledgments
Tirabrutinib has been approved on March 2020 for relapsed/refractory PCNSL in Japan. The authors wish to thank Keyra Martinez Dunn, MD, of Edanz Medical Writing, for providing medical writing support, which was funded by Ono Pharmaceutical Co, Ltd through EMC K.K., in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The authors would like to thank Professor Yasuo Iwadate (Department of Neurological Surgery, Chiba University, Chiba) and Dr Hirokazu Nagai (Department of Hematology and Oncology Research, Nagoya Medical Center, Nagoya) for their critical review of the clinical data as members of the Efficacy and Safety Monitoring Committee.
Conflict of interest statement. YN has received research funding and honoraria from Ono Pharmaceutical Co, Ltd for the submitted work; and research funding and honoraria from Chugai, AbbVie, Sumitomo Dainippon, Daiichi Sankyo, Eisai, Stella Pharmaceutical, Otsuka, Meiji Seika, and SBI Pharmaceuticals outside the submitted work. MN has received research funding and honoraria from Ono Pharmaceutical Co., Ltd. for the submitted work; and research funding and honoraria from Chugai, AbbVie, MSD, Eisai, Nippon Kayaku, Daiichi Sankyo, and UCB Japan; research funding from Otsuka, Pfizer, Astellas, Takeda, Tsumura, Shionogi, and Sanofi; advisory role fee from AbbVie, Daiichi-Sankyo, Sumitomo Dainippon, Bristol-Myers Squibb, and Riemser; honoraria from Novocure outside the submitted work. KM has received research funding from Ono Pharmaceutical Co, Ltd for the submitted work; and research funding from Chugai, MSD, Eisai, Teijin Pharma, Medical U and A, AbbVie, Otsuka, Daiichi Sankyo, and Nihon Medi-Physics outside the submitted work. YT has received research funding from Ono Pharmaceutical Co, Ltd for the submitted work. YA has received research funding from Ono Pharmaceutical Co., Ltd. for the submitted work; and research funding from Siemens, Philips, Sanofi, Nihon Medi-Physics, MitsubishiTanabe, Takeda, Stryker, Astellas Pharma, Taiho Pharma, and Pfizer; honoraria from Nippon Kayaku, Behring, AbbVie, Novocure, UCB Japan, Ono Pharmaceutical, and Otsuka; and research funding and honoraria from Brainlab, Merck, Chugai, Eisai, Meiji Seika, Daiichi Sankyo, Zeiss, and CLS outside the submitted work. HY has received research funding from Ono Pharmaceutical Co, Ltd for the submitted work. KA has received research funding from Ono Pharmaceutical Co, Ltd for the submitted work. NF has received research funding and honoraria from Ono Pharmaceutical Co, Ltd for the submitted work. KS has received research funding from Ono Pharmaceutical Co, Ltd for the submitted work; and research funding from Taiho, Daiichi Sankyo, Bristol-Myers Squibb, Chugai, Meiji Seika, and Yakult outside the submitted work. NS has received research funding from Ono Pharmaceutical Co, Ltd for the submitted work. JK and AA are employees of Ono Pharmaceutical Co, Ltd. RN has received research funding and honoraria from Ono Pharmaceutical Co, Ltd for the submitted work; and research funding from MSD; research funding and honoraria from AbbVie, Eisai, and Chugai; and honoraria from Novocure and Daiichi Sankyo outside the submitted work.
Authorship statement. All authors designed the study, interpreted the data, critically revised and provided final approval for the manuscript, and are accountable for the accuracy of its contents. JK and AA conceived the study; YN, MN, KM, YT, YA, HY, KA, NF, KS, NS, and RN participated in data acquisition; YN, JK, and AA drafted the manuscript.
References
- 1. Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92(2):261–266. [DOI] [PubMed] [Google Scholar]
- 2. Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Ann Oncol. 2007;18(11):1851–1855. [DOI] [PubMed] [Google Scholar]
- 3. Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21(6):1044–1049. [DOI] [PubMed] [Google Scholar]
- 4. Deckert M, Engert A, Brück W, et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia. 2011;25(12):1797–1807. [DOI] [PubMed] [Google Scholar]
- 5. Lin CH, Kuo KT, Chuang SS, et al. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006;12(4):1152–1156. [DOI] [PubMed] [Google Scholar]
- 6. Enblad G, Martinsson G, Baecklund E, et al. Population-based experience on primary central nervous system lymphoma 2000-2012: the incidence is increasing. Acta Oncol. 2017;56(4):599–607. [DOI] [PubMed] [Google Scholar]
- 7. Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5715. [DOI] [PubMed] [Google Scholar]
- 8. Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25(30):4730–4735. [DOI] [PubMed] [Google Scholar]
- 9. Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyakita Y, Ohno M, Takahashi M, Muragaki Y, Katai H, Narita Y. Immunochemotherapy using rituximab (RTX) and high-dose methotrexate (HD-MTX): an evaluation of the addition of RTX to HD-MTX in recurrent primary central nervous system lymphoma (PCNSL). Jpn J Clin Oncol. 2017;47(10):919–924. [DOI] [PubMed] [Google Scholar]
- 11. Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76(10):929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17(7):1141–1145. [DOI] [PubMed] [Google Scholar]
- 13. Korfel A, Schlegel U, Herrlinger U, et al. Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol. 2016;34(15):1757–1763. [DOI] [PubMed] [Google Scholar]
- 14. Rubenstein JL, Geng H, Fraser EJ, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018;2(13):1595–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soussain C, Hoang-Xuan K, Taillandier L, et al. ; Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26(15):2512–2518. [DOI] [PubMed] [Google Scholar]
- 16. Kasenda B, Ihorst G, Schroers R, et al. High-dose chemotherapy with autologous haematopoietic stem cell support for relapsed or refractory primary CNS lymphoma: a prospective multicentre trial by the German Cooperative PCNSL study group. Leukemia. 2017;31(12):2623–2629. [DOI] [PubMed] [Google Scholar]
- 17. Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833–843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130. [DOI] [PubMed] [Google Scholar]
- 22. Chamoun K, Choquet S, Boyle E, et al. Ibrutinib monotherapy in relapsed/refractory CNS lymphoma: a retrospective case series. Neurology. 2017;88(1):101–102. [DOI] [PubMed] [Google Scholar]
- 23. Berglöf A, Hamasy A, Meinke S, et al. Targets for ibrutinib beyond B cell malignancies. Scand J Immunol. 2015;82(3):208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107(29):13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walter HS, Rule SA, Dyer MJ, et al. A phase I clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127(4):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu J, Liu C, Tsui ST, Liu D. Second-generation inhibitors of Bruton tyrosine kinase. J Hematol Oncol. 2016;9(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yasuhiro T, Yoshizawa T, Daub H, Weber C, Narita M, Kawabata K. ONO-WG-307, a novel, potent and selective inhibitor of Bruton’s tyrosine kinase (Btk), results in sustained inhibition of the ERK, AKT and PKD signaling pathways [abstract]. Cancer Res. 2012;72(8 Suppl):2021. [Google Scholar]
- 29. Liclican A, Serafini L, Xing W, et al. Biochemical characterization of tirabrutinib and other irreversible inhibitors of Bruton’s tyrosine kinase reveals differences in on- and off-target inhibition. Biochim Biophys Acta Gen Subj. 2020;1864(4):129531. [DOI] [PubMed] [Google Scholar]
- 30. Munakata W, Ando K, Hatake K, et al. Phase I study of tirabrutinib (ONO-4059/GS-4059) in patients with relapsed or refractory B-cell malignancies in Japan. Cancer Sci. 2019;110(5):1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fukumura K, Kawazu M, Kojima S, et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol. 2016;131(6):865–875. [DOI] [PubMed] [Google Scholar]
- 32. Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279–290. [DOI] [PubMed] [Google Scholar]
- 33. Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332): 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoshizawa T, Yasuhiro T, Honda H, Kawabata K. ONO-4059—a potent and selective reversible Bruton’s tyrosine kinase (Btk) inhibitor: single agent, twice daily (BD) dosing and dosing with food results in sustained, high trough levels of ONO-4059, translating into 100% tumour remission in a TMD-8 xenograft model [abstract]. Blood. 2014;124(21):4502. [Google Scholar]
- 35. Bernard S, Goldwirt L, Amorim S, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126(14):1695–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lakshmanan A, Byrd JC. Spotlight on ibrutinib in PCNSL: adding another feather to its cap. Cancer Discov. 2017;7(9):940–942. [DOI] [PubMed] [Google Scholar]
- 37. Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–1679. [DOI] [PubMed] [Google Scholar]
- 38. Bartlett NL, Costello BA, LaPlant BR, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase II consortium trial. Blood. 2018;131(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bedsaul JR, Carter NM, Deibel KE, et al. Mechanisms of regulated and dysregulated CARD11 signaling in adaptive immunity and disease. Front Immunol. 2018;9:2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.