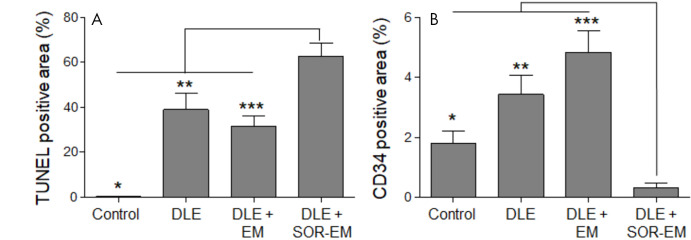
Figure 5:
A, B, Quantitative analysis of, A, terminal deoxynucleotidyl transferase-mediated dUTP (2’-deoxyuridine 5’-triphosphate) nick-end labeling (TUNEL)-positive (*P < .001, ** = .002, ***P < .001) and, B, human progenitor cell antigen (CD34)-positive areas (*P < .001, **P < .001, ***P < .001) in tumor tissue analyzed 2 weeks after the TACE treatment procedures (four rats per group). DLE = doxorubicin-Lipiodol emulsion, DLE + EM = DLE plus embolic microspheres, DLE + SOR-EM = DLE plus sorafenib-eluting EMs.