Table 2.
The anti-SARS-CoV-2 3CLpro activity of baicalein analogue flavonoids.
| Compound | Chemical structure | IC50 (μM) | % Inhibition at 50 μM |
|---|---|---|---|
| Scutellarein |
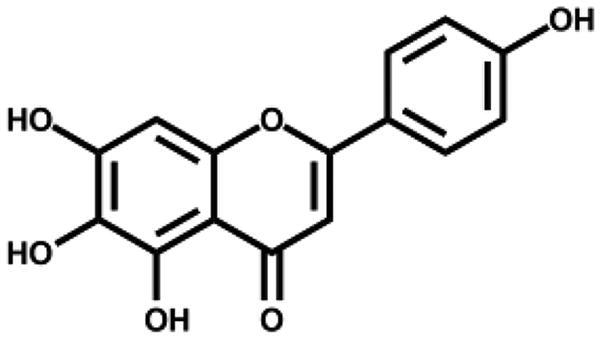
|
5.80 ± 0.22 | – |
| Dihydromyricetin |
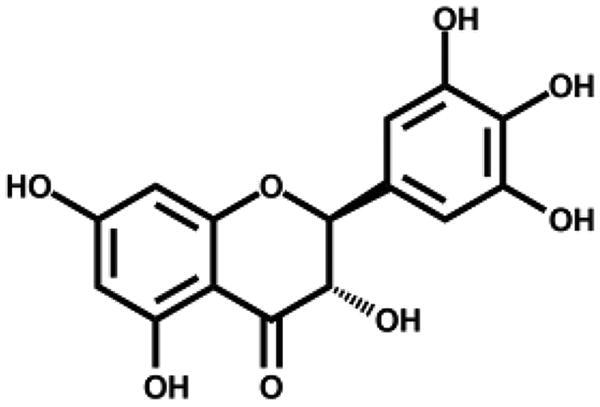
|
1.20 ± 0.09 | – |
| Quercetagetin |
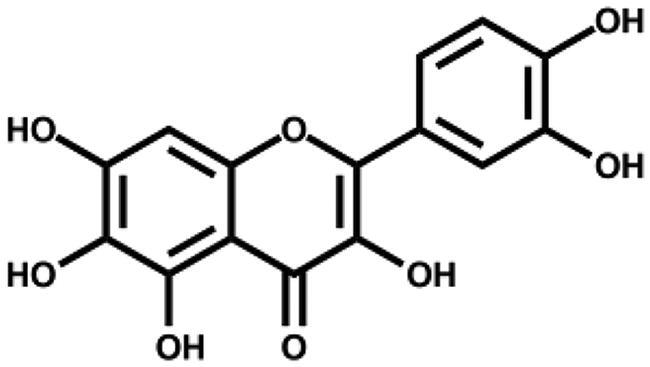
|
1.24 ± 0.14 | – |
| Myricetin |
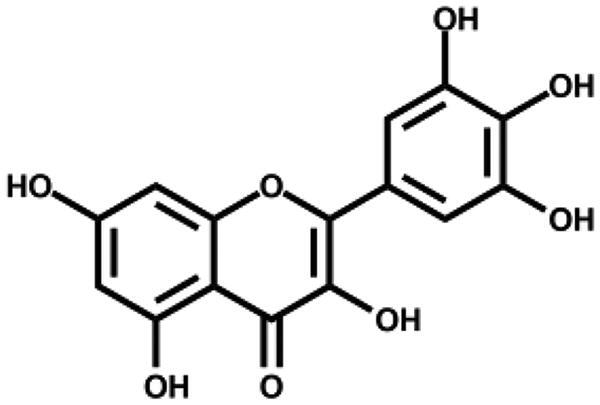
|
2.86 ± 0.23 | – |
| Scutellarin |
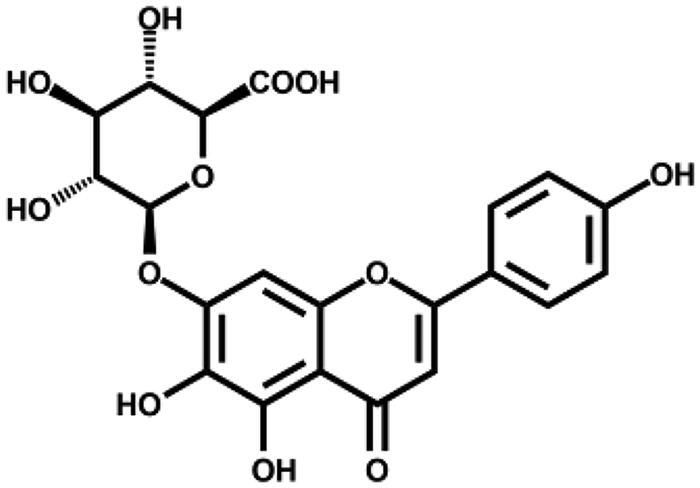
|
– | 28.9 ± 1.6 |
| 5,6-Dihydroxyflavone |
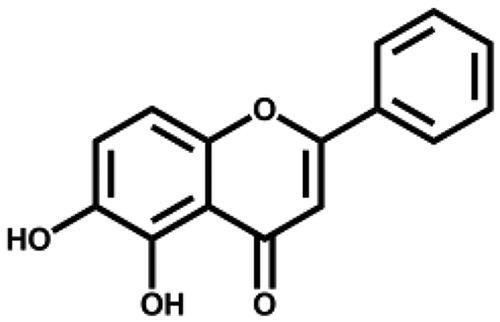
|
– | 26.6 ± 0.4 |
| 6,7-Dihydroxyflavone |
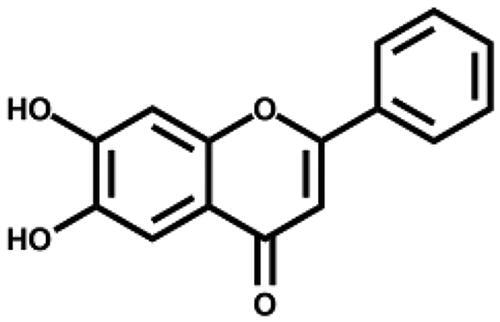
|
– | 56.7 ± 2.0 |
| Chrysin |
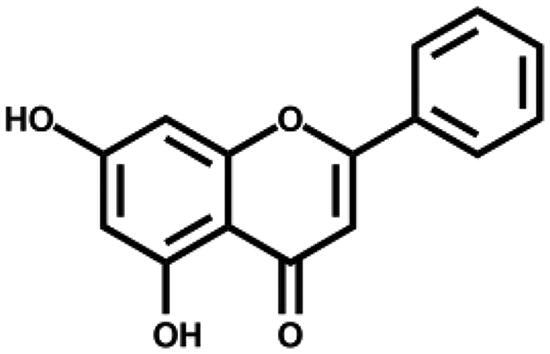
|
– | 2.6 ± 1.1 |
| Myricetin |
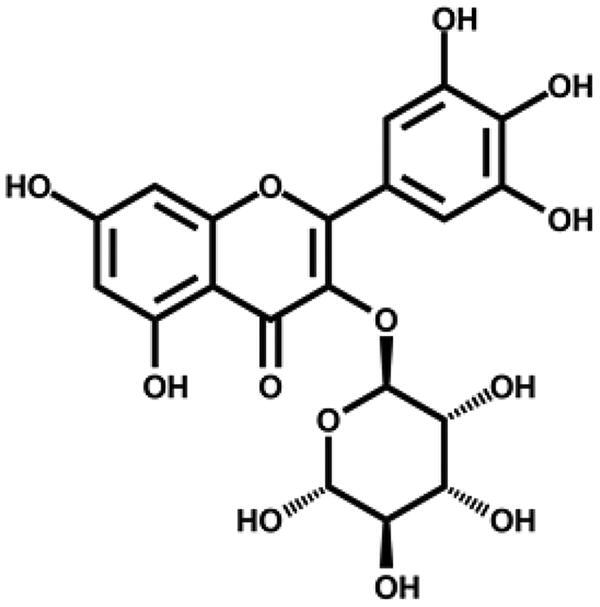
|
– | 30.8 ± 4.6 |
| Herbacetin |
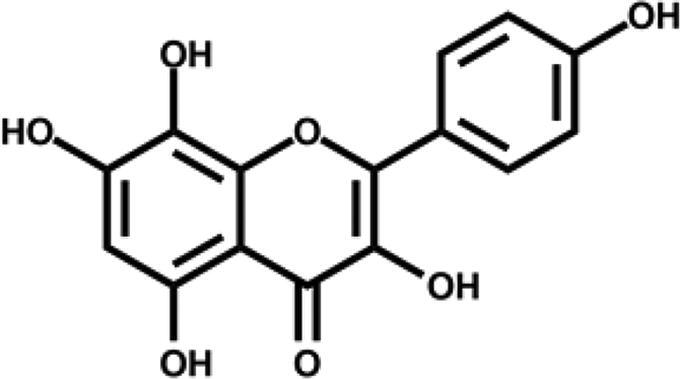
|
– | 59.1 ± 1.9 |
