Abstract
Background
Endocarditis, once predominately found in older adults, is increasingly common among younger persons who inject drugs. Untreated opioid use disorder (OUD) complicates endocarditis management. We aimed to determine if rates of overdose and rehospitalization differ between persons with OUD with endocarditis who are initiated on medications for OUD (MOUDs) within 30 days of hospital discharge and those who are not.
Methods
We performed a retrospective cohort study using a large commercial health insurance claims database of persons ≥18 years between July 1, 2010, and June 30, 2016. Primary outcomes included opioid-related overdoses and 1-year all-cause rehospitalization. We calculated incidence rates for the primary outcomes and developed Cox hazards models to predict time from discharge to each primary outcome as a function of receipt of MOUDs.
Results
The cohort included 768 individuals (mean age 39 years, 51% male). Only 5.7% of people received MOUDs in the 30 days following hospitalization. The opioid-related overdose rate among those who did receive MOUDs in the 30 days following hospitalization was lower than among those who did not (5.8 per 100 person-years [95% confidence interval [CI], 5.1–6.4] vs 7.3 per 100-person years [95% CI, 7.1–7.5], respectively). The rate of 1-year rehospitalization among those who received MOUDs was also lower than those who did not (162.0 per 100 person-years [95% CI, 157.4–166.6] vs 255.4 per 100 person-years [95% CI, 254.0–256.8], respectively). In the Cox hazards models, the receipt of MOUDs was not associated with either of the outcomes.
Conclusions
MOUD receipt following endocarditis may improve important health-related outcomes in commercially insured persons with OUD.
Keywords: endocarditis, opioid use disorder, opioid epidemic, medications, hospitalization
Health outcomes were improved when people with opioid use disorder who were hospitalized with endocarditis received medications for opioid use disorder (MOUDs). Compared with no MOUDs, receipt within 30 days of hospitalization was associated with reductions in overdose and rehospitalization.
(See the Editorial Commentary by Eaton on pages 479–81.)
BACKGROUND
An estimated 2.1 million people have an opioid use disorder (OUD) in the United States [1], although this may be an underestimate [2]. There has been a rise in injection drug use (IDU), specifically with nonprescription opioids such as heroin and synthetically produced fentanyl. Concurrently, mortality from infections and overdose has also risen [3].
Endocarditis is increasingly common among younger persons as complications of IDU [4–7]. Valve replacement is often necessary for endocarditis, which can result in prosthetic valve infections. An increasing proportion of mortality and cost associated with IDU is attributable to endocarditits, with hospitalization increases as high as 12-fold [4, 8, 9]. Endocarditis hospitalizations are lengthy [10] given the need for prolonged antibiotics. Rehospitalization for recurrent endocarditis and drug use-associated causes are frequent and costly [11].
Methadone, buprenorphine, and naltrexone are Food and Drug Adminisistration-approved medications for OUD (MOUDs) with evidence to support their effectiveness in improving mortality and retention in care [12–15]. Buprenorphine and methadone are especially beneficial at reducing opioid use, overdose, and death [16, 17]. Despite this, receipt of MOUDs during the peri-hospitalization period is uncommon [18, 19]. Common barriers include lack of training or knowledge [20], misperceptions about the feasibility of administering MOUDs [21], and limited resources for the transition to community-based treatment [22]. Hospitalization is a unique opportunity to initiate treatment and ensure linkage to care [23, 24]. It is unknown whether MOUD initiation during or upon discharge from an endocarditis hospitalization among persons with OUD improves outcomes. We aimed to determine if rates of health outcomes, including overdose and rehospitalization, differ between commercially insured persons with OUD hospitalized for endocarditis who are initiated on MOUDs in the peri-hospitalization period and those who are not initiated on MOUDs.
METHODS
Data Source
We analyzed data from the 2010–2016 MarketScan Commercial Claims and Encounters database, a nationally representative commercial insurance claims-based data set that includes ambulatory and inpatient visits, outpatient pharmacy claims, and diagnostic testing [25].
Inclusion Criteria
We selected individuals aged ≥18 years with OUD (Supplemental eTable 1) who were hospitalized for endocarditis between 2010 and 2016 and had a minimum 30-day follow-up after hospital discharge. We compared outcomes among those individuals who received MOUDs (Supplemental eTable 2) within 30 days of discharge for the index endocarditis to those who did not. We identified individuals with an initial inpatient claim for infective endocarditis (Supplemental eTable 3) using International Classification of Diseases, Ninth or Tenth Revisions (ICD-9 or ICD-10) codes. We included individuals who had a diagnosis of OUD either concurrent with or in the 6 months before or after the index endocarditis hospitalization [6]. OUD diagnosis was based on ICD-9/10 codes in inpatient or outpatient claims. We determined which codes to include based on expert opinion and previous literature [26]. We excluded individuals who had a pharmacy claim for MOUDs in the 3 months preceding their infection and individuals who had an ICD-9/10 diagnosis of concurrent stimulant use as treatment patterns differ in those patients.
Outcomes
The primary outcome measures included: (1) opioid-related overdose and (2) 1-year all-cause rehospitalization. We identified overdose events based on relevant ICD-9/10 codes. This outcome includes overdoses for which a medical claim was made, meaning a patient received care for an overdose at a hospital. We identified rehospitalization events using ICD-9/10 codes on inpatient claims.
Main Independent Variable
The primary independent variable was prescription for MOUD within 30 days of discharge. We used National Drug Codes to categorize MOUD and included: naltrexone (injectable extended release and oral formulations) and buprenorphine (mono- and coformulated with naloxone) [27]. Until late 2017, methadone therapy was not covered by commercial insurance; therefore, it is not reliably included in this data set. We are also not able to determine if MOUDs were initiated in the hospital.
We used outpatient prescription data to determine the date on which individuals filled their prescription following hospitalization and the days’ supply in each prescription. Individuals who filled a prescription within 4 weeks of discharge were categorized as being in the “MOUD” group. Individuals who did not fill a prescription or filled one after 4 weeks postdischarge were classified as “no MOUD.” In the time-to-event analyses, individuals began contributing follow-up time at discharge for their index endocarditis episode, and ceased contributing time when they encountered a primary outcome, were censored at the end of 1 year (in the case of rehospitalization) or at the end of the study period, or at exit from their insurance plan. The short-term intervention may not fully explain any differences in the long-term outcomes, and some individuals not immediately initiating MOUDs may receive an MOUD prescription later in the study period. To assess this, we performed a post hoc analysis to compare the average MOUD duration between those initiating MOUDs within 30 days of hospitalization and those who were prescribed MOUDs at a later date and who are grouped in our “no MOUD” sample.
Analyses
We calculated the overdose and all-cause rehospitalization incidence rates for the 2 groups: those who received MOUD treatment following hospitalization and those who did not. We calculated the total person-time and the total number of primary outcomes for each group. An overdose was counted at any point following the hospitalization through the end of the study, whereas rehospitalizations were limited to 1 year following index hospitalization. We calculated the incidence rates per 100 person-time and associated 95% confidence interval (CI) under the normality assumption. We also calculated the incidence rate of 30- and 90-day rehospitalization.
We developed weekly timescale Cox hazards models to predict time from hospital discharge to first overdose and first rehospitalization over the subsequent year as a function of receipt of MOUDs. The Cox models controlled for baseline demographic and clinical covariates including an individual’s sex, age, and region of residence and type of commercial insurance coverage; evidence of another substance use disorder during index hospitalization identified using ICD-9/10 codes (see Supplemental eTable 4), and whether or not cardiac surgery (eg, valve replacement) was performed during index hospitalization identified using ICD-9/10 and current procedural terminology (CPT) codes. The models adjusted for whether or not an individual had an interrupted hospitalization (defined as a break from discharge to readmission for endocarditis ≤10 days). Because this was based on expert opinion, we performed post hoc sensitivity analyses in which the hospitalization interruption was ≤5 and ≤30 days to account for uncertainty.
All statistical analyses were performed in SAS, version 9.4.
RESULTS
The cohort included 768 individuals with 978 person-years of follow-up. Baseline statistics of the cohort overall and by comparison group are presented in Table 1. The mean age was 39 years (standard deviation [SD] = 15.5), and 51% were male. The median length of hospitalization was 8 days (interquartile range [IQR] = 11), and 12% of this population underwent cardiac surgery during their index hospitalization associated with their endocarditis. Approximately 6% (44/768) of people received MOUDs in the 30 days following their index hospitalization for endocarditis. Those who received MOUDs were younger (mean age, 25 years ± 6.5) than those who did not receive MOUDs (40 years ± 15.5) (P < .0001). The mean MOUD prescription duration following discharge was 17.7 days (SD = 10.4 days). Buprenorphine was prescribed in 41 people of the 44 people who received MOUDs. Persons in the MOUD group (prescribed an MOUD within 30 days of hospitalization) had a longer average MOUD duration in the year following hospitalization than those in the “no MOUD” group who were prescribed an MOUD later in the year (9.7 weeks vs 8.6 weeks, P< .42).
Table 1.
Characteristics of Cohort of Individuals With OUD Who Were Hospitalized for Infective Endocarditis, 2010–2016
| Total | No MOUD | MOUD in 30 days | P value | |
|---|---|---|---|---|
| Overall number | 768 | 724 (94.3) | 44 (5.7) | |
| Age (mean years ± SD) | 39 (±15.5) | 40 (±15.5) | 25 (±6.5) | <.01 |
| Length of stay (median days, IQR) | 8 (11) | 8 (11) | 6 (9) | |
| Sex, n (%) | ||||
| Male | 394 (51.3) | 374 (51.7) | 20 (45.5) | .42 |
| Female | 374 (48.7) | 350 (48.3) | 24 (54.6) | |
| Region, n (%) | ||||
| Northeast | 193 (25.1) | 178 (24.6) | 15 (34.1) | .17 |
| North Central | 140 (18.2) | 132 (18.2) | 8 (18.2) | |
| South | 287 (37.4) | 277 (38.4) | 10 (22.7) | |
| West | 148 (19.3) | 137 (18.9) | 11 (25.0) | |
| Insurance type, n (%) | ||||
| HMO | 91 (11.9) | 82 (11.3) | 9 (20.5) | .32 |
| POS | 54 (7.0) | 52 (7.2) | 2 (4.6) | |
| PPO | 453 (59.0) | 429 (59.3) | 24 (54.6) | |
| Other | 170 (22.1) | 161 (22.2) | 9 (20.5) | |
| Cardiac surgery,a n (%) | ||||
| No | 674 (87.8) | 633 (87.4) | 41 (93.2) | .26 |
| Yes | 94 (12.2) | 91 (12.6) | 3 (6.8) | |
| Other substance use disorders,b n (%) | ||||
| No | 713 (92.8) | 674 (93.1) | 39 (88.6) | .27 |
| Yes | 55 (7.2) | 50 (6.9) | 5 (11.4) |
Abbreviations: HMO, health maintenance organization; ICD, International Classification of Diseases, Ninth or Tenth Revisions; IQR, interquartile range; MOUD, medications for OUD; OUD, opioid use disorder; POS, point of service; PPO, preferred provider organization; SD, standard deviation.
aComplete list of ICD-9 and ICD-10 codes found in Supplemental eTable 3.
bDefined as evidence of another substance use disorder (including alcohol, antidepressants, cannabis, hallucinogens, or sedatives). Complete list of ICD-9 and ICD-10 codes found in Supplemental eTable 4.
Overdose Rates
We found 41 overdoses among those who did not receive MOUDs, leading to a rate of 7.3 overdoses per 100-person years (95% CI, 7.1–7.5). Comparatively, there was a rate of 5.8 overdoses per 100 person-years (95% CI, 5.1–6.4) among those who did receive MOUDs.
Rehospitalization Rates
There was a significant difference in 1-year rehospitalization rates between the 2 groups. The rate of 1-year rehospitalization among those who did not receive MOUDs was 255.4 per 100 person-years (95% CI, 254.0–256.8), and for those who did was 162.0 per 100 person-years (95% CI, 157.4–166.6). The rate of 30-day rehospitalization among those who did not receive MOUDs was 40.5 per 100 person-30 days (95% CI, 40.0–40.9) and for those who did was 32.6 per 100 person-30 days (95% CI, 30.9–34.3). The rate of 90-day rehospitalization among those who did not receive MOUDs was 85.8 per 100 person-90 days (95% CI, 85.1–86.5) and for those who did was 59.5 per 100 person-90 days (95% CI, 57.1–61.9).
Cox-Adjusted Models
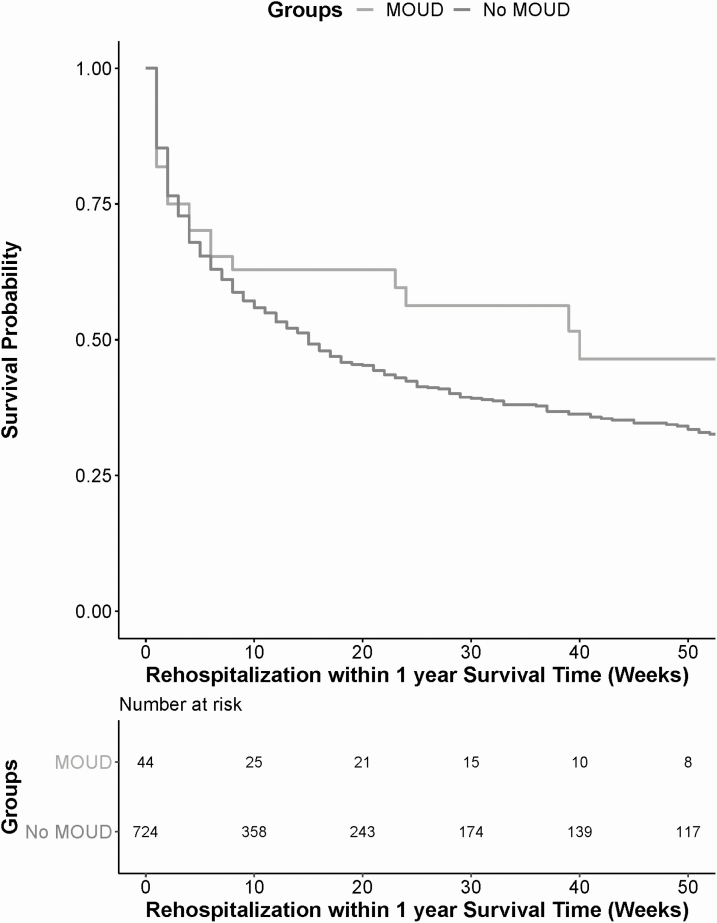
Without controlling for covariates, there was not a significant risk reduction in overdose for the MOUD group compared to the “no MOUD” group (hazard ratio [HR] = 1.18; 95% CI, .36–3.80). There was a risk reduction in 1-year rehospitalization for the MOUD group compared to the “no MOUD” group that approached significance (HR = .71, 95% CI, .45–1.11) (Figure 1). In the adjusted models, the receipt of MOUDs was not associated with overdose (Table 2) or 1-year all-cause rehospitalization (Table 3) (adjusted hazard ratio [aHR] of overdose = 0.86; 95% CI, .26–2.91; aHR for 1-year rehospitalization = 0.81; 95% CI, .51–1.28). The results were not qualitatively different in the sensitivity analyses in which the hospitalization interruption was ≤5 days (overdose: aHR = 0.80; 95% CI, .24–2.68; 1-year rehospitalization: aHR = 0.76; 95% CI, .48–1.21) or when it was ≤30 days (overdose: aHR = 0.84; 95% CI, .25–2.84; 1-year rehospitalization: aHR = 0.84; 95% CI, .53–1.34).
Figure 1.
Kaplan–Meier curve of 1-year rehospitalization for persons who received MOUDs following hospitalization compared to no MOUDs. Time-to-event analysis for 1-year rehospitalization compared individuals with infective endocarditis who received MOUDs in the peri-hospitalization period to those who did not receive MOUDs. Without controlling for covariates, those who received MOUDs (gray line) had a lower risk of one-year rehospitalization than those who did not receive MOUDs (black line). Abbreviation: MOUD, medication for opioid use disorder.
Table 2.
Results of Cox Hazards Model for Opioid-Related Overdose
| Parameter | Adjusted Hazard Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|
| Treatment | ||||
| MOUD within 4 weeks | 0.86 | .26 | 2.91 | .81 |
| No MOUD within 4 weeks | Reference | |||
| Clinical characteristics | ||||
| Other substance use disordera | ||||
| Yes | 0.86 | .26 | 2.84 | .81 |
| No | Reference | |||
| Cardiac surgeryb | ||||
| Yes | 0.44 | .15 | 1.32 | .14 |
| No | Reference | |||
| Hospital interruptionc | ||||
| Yes | 3.56 | 1.61 | 7.88 | <.01 |
| No | Reference | |||
| Nonclinical characteristics | ||||
| Age (years) | 0.97 | .95 | 0.99 | .01 |
| Sex | ||||
| Male | Reference | |||
| Female | 0.89 | .47 | 1.63 | .68 |
| Region | ||||
| Northeast | Reference | |||
| North Central | 0.87 | .40 | 1.86 | .71 |
| South | 0.37 | .16 | 0.85 | .02 |
| West | 0.36 | .14 | 0.93 | .04 |
| Insurance type | ||||
| POS | Reference | |||
| PPO | 1.50 | .44 | 5.12 | .52 |
| HMO | 1.12 | .27 | 4.61 | .87 |
| Other | 1.77 | .47 | 6.59 | .40 |
Abbreviations: CPT, current procedural terminology; HMO, health maintenance organization; ICD, International Classification of Diseases, Ninth or Tenth Revisions; MOUD, medication for opioid use disorder; POS, point of service; PPO, preferred provider organization.
aDefined as evidence of another substance use disorder (including alcohol, cannabis, hallucinogens, or sedatives). Complete list of ICD-9 and ICD-10 codes found in Supplemental eTable 4.
bComplete list of ICD-9, ICD-10, and CPT codes found in Supplemental eTable 3.
cInterruption of index hospitalization ≤10 days.
Table 3.
Results of Cox Hazards Model for 1-Year Rehospitalization
| Parameter | Adjusted Hazard Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|
| Treatment | ||||
| MOUD within 4 weeks | 0.81 | .51 | 1.28 | .36 |
| No MOUD within 4 weeks | Reference | |||
| Clinical characteristics | ||||
| Other substance use disordera | ||||
| Yes | 1.11 | .78 | 1.59 | .55 |
| No | Reference | |||
| Cardiac surgeryb | ||||
| Yes | 1.01 | .76 | 1.34 | .96 |
| No | Reference | |||
| Hospital interruptionc | ||||
| Yes | 5.49 | 4.00 | 7.52 | <.01 |
| No | Reference | |||
| Nonclinical characteristics | ||||
| Age (years) | 1.01 | 1.00 | 1.01 | .05 |
| Sex | ||||
| Male | Reference | |||
| Female | 1.18 | .98 | 1.43 | .08 |
| Region | ||||
| Northeast | Reference | |||
| North Central | 0.96 | .71 | 1.28 | .76 |
| South | 1.00 | .78 | 1.27 | .97 |
| West | 1.00 | .81 | 1.33 | 1.00 |
| Insurance type | ||||
| POS | Reference | |||
| PPO | 1.20 | .81 | 1.78 | .37 |
| HMO | 1.12 | .70 | 1.78 | .65 |
| Other | 1.16 | .76 | 1.56 | .48 |
Abbreviations: CPT, current procedural terminology; HMO, health maintenance organization; ICD, International Classification of Diseases, Ninth or Tenth Revisions; MOUD, medication for opioid use disorder; POS, point of service; PPO, preferred provider organization.
aDefined as evidence of another substance use disorder (including alcohol, cannabis, hallucinogens, or sedatives). Complete list of ICD-9 and ICD-10 codes found in Supplemental eTable 4.
bComplete list of ICD-9, ICD-10, and CPT codes found in Supplemental eTable 3.
cInterruption of index hospitalization ≤10 days.
Several covariates were examined to determine whether they were predictive of experiencing an overdose or rehospitalization. Persons who had a ≤10 or ≤30 day interruption in their index hospitalization were at increased risk compared to those who did not have an interruption for overdose (aHR = 3.56, 95% CI, 1.61–7.88, and aHR = 2.46, 95% CI, 1.12–45.39, respectively) and 1-year rehospitalization (aHR = 5.49, 95% CI, 4.00–7.52, and aHR = 5.47, 95% CI, 4.15–7.22, respectively). Persons who underwent cardiac surgery were not more likely to overdose (aHR = 0.44; 95% CI, .15–1.32) or be rehospitalized at 1 year (aHR = 1.01; 95% CI, .76–1.34) than those without surgery.
DISCUSSION
In this analysis of commercially insured individuals with OUD and endocarditis, we found that while overall receipt of MOUDs following hospitalization for endocarditis was low, those who did receive MOUDs had lower incidence of overdose and 1-year rehospitalization than those who did not. This study suggests that incorporating MOUDs into the treatment paradigm for endocarditis may improve outcomes. It may also stand to reason that integrating MOUDs into the treatment paradigm for other infections related to opioid use may also improve outcomes. The mechanism being that MOUDs lead to decreased injection use, thus decreasing the likelihood of introducing bacteria or fungus into the body.
Overall, only 5.7% of persons in this cohort received MOUDs in the 30 days following hospitalization for endocarditis. This is consistent with other data detailing MOUD receipt following endocarditis [18] but lower than other studies examining MOUD receipt among people in contact with the healthcare system. LaRochelle and colleagues found that nearly 30% of people who experience a nonfatal overdose received MOUDs in the year following hospitalization [28]. Hadland et al [29] found that approximately 25% of youth ages 13–22 received a MOUD within 3 months of their OUD diagnosis. These studies demonstrate the infrequency of MOUD prescribing, despite the worsening drug overdose epidemic and increased awareness of the efficacy of MOUDs.
The recent increasing endocarditis prevalence among people who use drugs (PWID) has been well described [7, 10, 30]. These studies show that a larger proportion of cases of endocarditis are among PWID, which is, in turn, lowering the overall average age of this infection. This has implications for healthcare costs and long-term patient outcomes. For example, because valve replacement is a common component of endocarditis treatment, younger age at valve replacement lengthens the exposure period for risks associated with prosthetic valves, which can be costly and highly morbid. Other studies have compared the clinical outcomes among those with endocarditis who do and not use drugs. Rudasill et al [11] examined readmission rates among people with IDU-associated infective endocarditis (IDU-IE) and non-IDU-IE and found no significant differences between the 2 groups. Importantly, the authors did not examine outcomes stratified by MOUD receipt in the IDU-IE group.
Our study is among the first to our knowledge to demonstrate improvement in crucial outcomes—opioid overdose and 1-year rehospitalization—when treatment for endocarditis includes peri-hospitalization MOUD. In our study, healthcare utilization was assessed over a short period of time (1-year) because peri-hospitalization MOUDs may not have an impact on rehospitalization at a more distal time point. Overdose was assessed at any point following hospitalization because MOUDs do change the trajectory of OUD and may impact overdose at distal time points. Our findings argue that the peri-hospitalization period for a serious infections may be 1 high-value time point for MOUD initiation. MOUDs and addiction treatment should be considered essential components of treatment for injection-related infections. If the underlying substance use disorder remains untreated, patients are likely to experience poor outcomes.
Although the receipt of peri-hospitalization MOUDs did lower the incidence rates, it did not have an impact on the adjusted time-to-event analyses for either outcome. This may be attributed to low overall event occurrences. Another plausible explanation for this finding is the lack of methadone treatment information in the claims database. Because we were unable to determine whether or not an individual was prescribed methadone following hospitalization, those individuals were therefore included in the “no MOUDs” group. Existing data are scarce regarding use of methadone and buprenorphine within the same year to treat OUD. One study that used the National HIV Behavioral Surveillance system found that 1.8% of persons had used both medications in the previous year [31]. This provides some supporting evidence that persons who were treated with methadone in our cohort are unlikely to also have been treated with buprenorphine in the same year. Although this is a limitation of the data set, the inclusion of persons who received methadone in the “no MOUD” group would have biased our result toward the null hypothesis and against the effectiveness of MOUDs. Analyses of publicly insured individuals might therefore have different results than ours.
There were significant findings among the covariates. Notably, we found that hospitalization interruption was associated with an increased risk of primary outcomes. The 10-day interruption window was selected to model a possible discharge against medical advice (increasingly being referred to as “patient directed discharge”) with then a return to the hospital for continued treatment for endocarditis. In the sensitivity analyses in which the hospital interruption was ≤5 and ≤30 days, the results did not qualitatively change. Often, treatment interruptions or discharges against medical advice are the result of untreated opioid withdrawal or cravings. As such, efforts to avoid these treatment interruptions should include inpatient initiation of MOUDs. We also found that geographic region, specifically the West and South, were associated with decreased risk of overdose compared to the Northeast. This may be explained by fentanyl that was present throughout much of the study in the Northeast but not in the West or the South [32].
Although it is encouraging that MOUDs improved outcomes, OUD is a chronic relapsing disease, and 1-time receipt of medications is but 1 component of treatment. Many people experience multiple relapses and reinitiate MOUDs numerous times. Short treatment durations such as those noted in this study are unlikely to promote sustained recovery [33]. Much like the continua of care for other chronic diseases like human immunodeficiency virus and hepatitis C virus, efforts are needed to improve retention in care. One potential approach is to develop a comprehensive inpatient treatment package at the time of endocarditis that includes infectious diseases and addiction medicine consultations, initiation of MOUD, linkage services to outpatient addiction care, and social work involvement to help address underlying social and structural issues such as homelessness, untreated mental illness, and co-occurring substance use disorders that are often barriers to retention and recovery [19]. Additionally, an integrated approach in the outpatient setting that involves colocated treatment for both the substance use disorder and the drug use-associated infection would also likely improve outcomes [34–36]. A first crucial step is to improve low barrier access to MOUD as early initiation of MOUD should be standard of care for persons with OUD-related infections.
In addition to the discussion of methadone, there were some limitations that merit discussion. First, as a commercial claims database, Marketscan does not include individuals who are uninsured or on public insurance (eg, Medicaid). Inadequate health insurance coverage for substance use services can be a barrier to treatment. In the years following the implementation of the Patient Protection and Affordable Care Act, the odds of being uninsured among persons with heroin use disorder decreased by 40%, largely due to an increase in prevalence of Medicaid coverage [37]. In fact, uninsured rates for individuals with OUD are close to commercially insured rates, our study population [38]. Thus, a large proportion of people who inject opioids are publicly insured [39] rather than uninsured. Studies have shown that OUD treatment length among persons on Medicaid are similar to commercially insured persons [40, 41]. Nonetheless, there may be differences between the commercially insured, publicly insured, and uninsured populations regarding MOUD dosing or posthospital linkage and access to care that may affect the outcomes and limit generalizability. Additionally, we are unable to evaluate associations with overdose fatalities as we cannot identify whether an overdose was fatal since individuals may exit the data set due to death or when they dis-enrolled from an employer sponsored insurance plan. Finally, although a growing problem in the United States, we are not able to fully explore polysubstance overdose given the lack of toxicology in claims data.
In conclusion, this analysis using a commercial claims data set suggested an impact of MOUDs in the peri-hospitalization period for endocarditis among people with OUD. Addressing OUD is a key element of comprehensive care. More work is need to integrate care for infections associated with drug use that address the infection, the OUD, and the structural issues that inhibit retention in care and long-term recovery.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to thank Dr. Rich Saitz for his thoughtful comments and guidance on the content of this paper.
Financial support. This work was supported by Charles A King Trust Award to J. A. B.; the National Institute on Drug Abuse at the National Institutes of Health (R01DA046527 to J. A. B, J. W., J. R. M., and P30DA040500 to J. R. M.); and Tufts University School of Medicine (KL2TR002545-01 to A. W.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 2017. [Google Scholar]
- 2. Barocas JA, White LF, Wang J, et al. Estimated prevalence of opioid use disorder in Massachusetts, 2011–2015: a capture-recapture analysis. Am J Public Health 2018; 108:1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011; 60:1487–92. [PubMed] [Google Scholar]
- 4. Fleischauer AT, Ruhl L, Rhea S, Barnes E. Hospitalizations for endocarditis and associated health care costs among persons with diagnosed drug dependence—North Carolina, 2010–2015. MMWR Morb Mortal Wkly Rep 2017; 66:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray ME, Rogawski McQuade ET, Scheld WM, Dillingham RA. Rising rates of injection drug use associated infective endocarditis in Virginia with missed opportunities for addiction treatment referral: a retrospective cohort study. BMC Infect Dis 2018; 18:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug-related infections are missed: a population-based cohort study of inpatient and emergency department settings. Clin Infect Dis 2019; 68:1166–75. [DOI] [PubMed] [Google Scholar]
- 7. Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3): ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ciccarone D, Unick GJ, Cohen JK, Mars SG, Rosenblum D. Nationwide increase in hospitalizations for heroin-related soft tissue infections: associations with structural market conditions. Drug Alcohol Depend 2016; 163:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood) 2016; 35:832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collier MG, Doshani M, Asher A. Using population based hospitalization data to monitor increases in conditions causing morbidity among persons who inject drugs. J Community Health 2018; 43:598–603. [DOI] [PubMed] [Google Scholar]
- 11. Rudasill SE, Sanaiha Y, Mardock AL, et al. Clinical outcomes of infective endocarditis in injection drug users. J Am Coll Cardiol 2019; 73:559–70. [DOI] [PubMed] [Google Scholar]
- 12. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies: tackling the opioid-overdose epidemic. N Engl J Med 2014; 370:2063–6. [DOI] [PubMed] [Google Scholar]
- 13. Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet 2003; 361:662–8. [DOI] [PubMed] [Google Scholar]
- 14. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; ( 2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med 2015; 9:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hedrich D, Alves P, Farrell M, Stöver H, Møller L, Mayet S. The effectiveness of opioid maintenance treatment in prison settings: a systematic review. Addiction 2012; 107:501–17. [DOI] [PubMed] [Google Scholar]
- 17. Larney S, Gisev N, Farrell M, et al. Opioid substitution therapy as a strategy to reduce deaths in prison: retrospective cohort study. BMJ Open 2014; 4:e004666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med 2016; 129:481–5. [DOI] [PubMed] [Google Scholar]
- 19. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med 2014; 174:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cunningham CO, Sohler NL, McCoy K, Kunins HV. Attending physicians’ and residents’ attitudes and beliefs about prescribing buprenorphine at an urban teaching hospital. Fam Med 2006; 38:336–40. [PubMed] [Google Scholar]
- 21. Hassamal S, Goldenberg M, Ishak W, Haglund M, Miotto K, Danovitch I. Overcoming barriers to initiating medication-assisted treatment for heroin use disorder in a general medical hospital: a case report and narrative literature review. J Psychiatr Pract 2017; 23:221–9. [DOI] [PubMed] [Google Scholar]
- 22. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med 2018; 13:62–4. [DOI] [PubMed] [Google Scholar]
- 23. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med 2010; 25:803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med 2017; 32:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naeger S, Mutter R, Ali MM, Mark T, Hughey L. Post-discharge treatment engagement among patients with an opioid-use disorder. J Subst Abuse Treat 2016; 69:64–71. [DOI] [PubMed] [Google Scholar]
- 26. Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend 2019; 200:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Micromedex Solutions. 2018. Drug Topics Red Book. online [Internet]. Available at: http://www.micromedexsolutions.com. Accessed 24 June 2019.
- 28. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med 2018; 169:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hadland SE, Bagley SM, Rodean J, et al. Receipt of timely addiction treatment and association of early medication treatment with retention in care among youths with opioid use disorder. JAMA Pediatr 2018; 172:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med 2018; 170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsui JI, Burt R, Thiede H, Glick SN. Utilization of buprenorphine and methadone among opioid users who inject drugs. Subst Abus 2018; 39:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zoorob M Fentanyl shock: the changing geography of overdose in the United States. Int J Drug Policy 2019; 70:40–6. [DOI] [PubMed] [Google Scholar]
- 33. Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat 2009; 37:426–30. [DOI] [PubMed] [Google Scholar]
- 34. Barocas JA, Morgan JR, Fiellin DA, et al. Cost-effectiveness of integrating buprenorphine-naloxone treatment for opioid use disorder into clinical care for persons with HIV/hepatitis C co-infection who inject opioids. Int J Drug Policy 2019; 72:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gutkind S, Schackman BR, Morgan JR, et al. Cost-effectiveness of HCV treatment models for people who inject drugs in opioid agonist treatment programs. Clin Infect Dis 2019. Available at: https://www.ncbi.nlm.nih.gov/pubmed/31095683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Altice FL, Bruce RD, Lucas GM, et al. ; BHIVES Collaborative HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr 2011; 56(Suppl 1):S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feder KA, Mojtabai R, Krawczyk N, et al. Trends in insurance coverage and treatment among persons with opioid use disorders following the Affordable Care Act. Drug Alcohol Depend 2017; 179:271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKenna RM Treatment use, sources of payment, and financial barriers to treatment among individuals with opioid use disorder following the national implementation of the ACA. Drug Alcohol Depend 2017; 179:87–92. [DOI] [PubMed] [Google Scholar]
- 39. Peterson C, Xu L, Mikosz CA, Florence C, Mack KA. US hospital discharges documenting patient opioid use disorder without opioid overdose or treatment services, 2011–2015. J Subst Abuse Treat 2018; 92:35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat 2018; 95:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saloner B, Daubresse M, Caleb Alexander G. Patterns of buprenorphine-naloxone treatment for opioid use disorder in a multistate population. Med Care 2017; 55:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.