Figure 1.
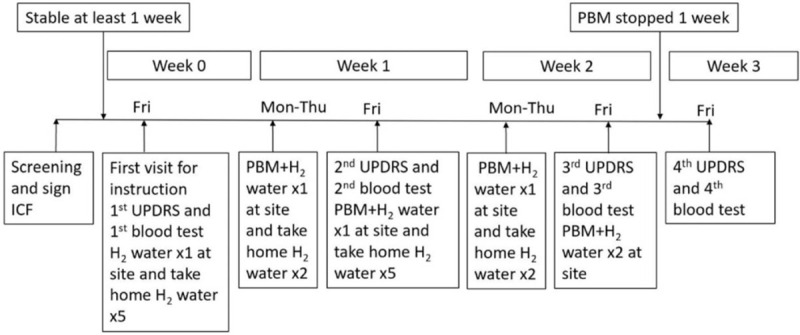
Scheme of the study design. Participants fulfill the criteria of inclusion and exclusion, the ICF of this study was explained and signed. After a week of stable condition (without any change of anti-PD medications or other active medical conditions), the participant will visit our study site for detail instructions of the study on Friday of Week 0. The first UPDRS was scored and blood was drawn for laboratory study. Hydrogen water was supplied, including those to be used during weekend at home. On Week 1 and Week 2, through Monday to Friday, the participant received daily PBM and hydrogen water at study site under the help of our study assistant. On each Friday of Week 1 and 2, UPDRS was recorded and blood sample was collected. On Week 3, the PBM and hydrogen water consumption was ceased. Another UPDRS score and blood sample was collected on Friday before the end of the study. ICF = informed consent form, PBM = photobiomodulation, UPDRS = Unified Parkinson Disease Rating Scale.
