Abstract
According to the analysis to find out how demographic and clinical characteristics influent the dysphagia outcome after stroke, furthermore, giving some insights to clinical treatment.
One hundred eighty post-stroke dysphagia (PSD) patients were enrolled in this retrospective study at the stroke rehabilitation department. The outcome measurements are beside water swallow test at discharge and length of stay at hospital. Twenty-five demographic and clinical variables were collected for this study. Logistic regression and multilinear regression were utilized to estimate models to identify the risk and protect predictors of PSD outcome.
Mouth-opening degree, drooling severity scale (DSS) level, mini-mental state exam (MMSE) level, Barthel index and Berg balance scale were significant different between recovered and unrecovered group. Type of stroke, MMSE degree, DSS and hemoglobin level shown significant predictive value for PSD outcome in logistic regression. In addition, obstructive sleep apnea (OSA) and DSS degree were important risk factors for PSD outcome. Gender, body mass index, drinking, hypertension, recurrent stroke, water swallow test level on admission, Berg balance scale, DSS and days between onset to admission shown significant predictive value for length of stay of PSD patients.
PSD outcome was influenced by type of stroke, MMSE degree, DSS and hemoglobin level significantly and obstructive sleep apnea act as an important risk role for PSD recovery.
Keywords: dysphagia, recovery, regression, stroke
1. Introduction
With the development of post-stroke treatment technology, about 84% of patients can survive from the acute phase.[1] However, stroke is still the second leading cause of death worldwide after ischemic heart disease.[2] Dysphagia is one of the common symptoms of stroke patients and worsen post-stroke outcome.[3] It has been reported that pay attention on post-stroke dysphasia earlier might decrease the length of stay at hospital, morbidity and mortality.[3] Several studies have investigated the risk factors for presenting dysphagia or demonstrate dysphagia is one of the risk factors for adverse outcome. The risk factors for failing the dysphagia test including old age, female, atrial fibrillation, lower Barthel score, visual field defect, aphasia, weakness, admission to intensive care unit.[4] Pneumonia, discharge to long-term care, severe disability, 1-year mortality have been considered as dysphagia related complications to worsen outcome.[5] Hinchey reveled that the evaluation and management of dysphasia early might be an efficient way to reduce dysphasia complications and improve the stroke outcome.[6]
The management of PSD including modify food and fluid, alter to a safe posture and some rehabilitative techniques.[7] However, no good evidence was found to reveal that modified food to thickening liquids can decrease dysphagia related pneumonia.[8] Furthermore, the nutrition of these kind of food probably not enough to support the patients who suffered an acute severe wasting disease.[9] Therefore, the efficiency of this method to be a kind of treatment for PSD is unknown. Alter to 45° reclining sitting posture has been revealed to be a beneficial way to avoid aspiration for patients with a mass of residue or a small amount of penetration or aspiration.[10] Although, several studies have been focused on the body position for PSD patients, there is no safe posture effective for everyone. So, it needs to be individualization. Some rehabilitation techniques have been supported for PSD including Shaker exercise,[10] proprioceptive neuromuscular facilitation,[11] electrical pharyngeal stimulation whereas some have been evidenced ineffective.[12] Overall, the treatment methods for PSD are limited.
Therefore, identification and management of risk factors for post-stroke dysphagia recovery might be an efficient method to improve the dysphagia outcome. However, few studies were found to investigate how demographic and clinical characteristics contribute to the post-stroke dysphagia outcome. So, the aim of this study is to discover the risk factors for PSD recovery. The hypothesized is that many risk factors might present overlap with stroke recovery due to dysphasia is part of symptoms of stroke but still some specific factors exist for swallowing function.
2. Methods
2.1. Participants
This study was approved by Institutional Review Board and all participants signed informed consent in the study. Inclusion criteria and exclusion criteria contains
-
1)
confirmed stroke by computed tomography or/and magnetic resonance imaging,
-
2)
measured dysphagia by bedside water swallow test (WST) primarily and videofluoroscopic Swallow study eventually except who is not appropriate to do this test, such as using mechanical ventilator, iodine allergy,
-
3)
more than 1 week treatment,
-
4)
without other diseases which might affect swallowing function, such as motor neuron disease, myasthenia gravis, Parkinson disease,
-
5)
hemorrhagic transformation after infarction and stroke located in both sites are excluded. Demographic and clinical characteristics including gender, age, body mass index (BMI), smoking, drinking, diabetes mellitus, hypertension, coronary artery disease (CAD), atrial fibrillation, obstructive sleep apnea (OSA), recurrent stroke, type of stroke, stroke location, disorder of consciousness, tracheotomy, hemoglobin (HB) and albumin on admission were extracted from the medical record.
2.2. Outcome measure
WST is the primary outcome measurement which is an efficient method to give a quick assessment on swallow function. WST contains 5 levels and the first level representing normal swallow, fifth level indicating the most sever dysphagia. WST was conducted on admission and at discharge. Participants was grouped into 2 groups by the WST level at discharge, which level 1 was considered as recovered, others are unrecovered. The second outcome measurement is length of stay (LOS) at this rehabilitation department.
2.3. Stroke functional measurement
The Patient Health Questionnaire-9 which is considered as a reliable and valid measurement for depression[13] was conducted to every participant on admission. The mini-mental state exam (MMSE) was employed to measure the cognition which is widely used for evaluating cognitive problems. It contains 30 items measuring function about orientation, attention, learning, calculation, delayed recall, and construction. Barthel index (BI) was used to assess the activity of daily life which is considered as a reliable evaluation tool for stroke patients.[14] Berg balance scale (BBS) is an evaluation of motor function, it has been reported as a valid measurement to evaluate the balance and functional mobility for post-stroke individuals.[15]
2.4. Dysphagia functional measurement
To assess the mouth opening degree, the functional staging/classification: Based on mouth opening between upper and lower central incisors method was utilized which is apply for assessment of oral submucous fibrosis usually.[16] It contains 5 stages: stage I, maximum interincisal mouth opening up to or >35 mm; stage II, maximum interincisal mouth opening between 25 and 35 mm; stage III, maximum interincisal mouth opening between 15 and 25 mm; stage IV, maximum interincisal mouth opening between 5 and 15 mm; stage V, maximum interincisal mouth opening < 5 or nil. The severity part of the drooling severity scale (DSS) was used to measure the drooling of PSD giving a score range from 1 (normal) to 5 (severe).
2.5. Statistics
2.5.1. Univariate analyses
Two sample t test was used to compare continuous data, and Chi-squared test was conducted for categories data. P < .05 was considered as significant difference. All analysis was performed by R (3.6.1).
2.5.2. Estimation of regression model
The following variables were employed to give a prediction of dysphasia outcome: gender, age, BMI, smoking, drinking, diabetes mellitus, hypertension, CAD, atrial fibrillation, OSA, recurrent stroke, type of stroke, stroke location, disorder of consciousness, tracheotomy, depression, HB, albumin, MMSE level, BI, BBS, mouth-opening degree and DSS. glm and lm functions were applied to compute the logistic regression model (for the outcome of recovered and unrecovered) and linear regression model (for the outcome of LOS), respectively. Furthermore, Forest plot was used to display the two regression models by the ggforestplot (0.0.2) package of R.
3. Results
3.1. Clinical characteristics
One hundred eighty post-stroke dysphagia patients were enrolled in this study eventually from February 2017 to December in 2018 at the rehabilitation department. Participants was grouped into 2 groups by the WST level at discharge. Demographical and clinical characteristics differences between 2 groups were shown as Table 1. Significant differences were found in mouth-opening degree, DSS level, MMSE level, Barthel index and Berg balance scale (P < .05).
Table 1.
Demographical and clinical characteristics.
| Recovered (n = 90) | Unrecovered (n = 90) | P | |
| Gender | Male (62, 68.9%) | Male (64,71.1%) | .871 |
| Age | 65.1 ± 11.5 | 63.9 ± 11.7 | .495 |
| BMI | 22.2 ± 3.01 | 21.5 ± 3.21 | .064 |
| Risk factors | Smoking (30, 33.3%) | Smoking (32,35.6%) | .745 |
| Drinking (20, 22.2%) | Drinking (22, 24.4%) | .732 | |
| DM (24, 26.7%) | DM (22, 24.4%) | .865 | |
| Hypertension (56, 62.2%) | Hypertension (58, 64.4%) | .864 | |
| CAD (14, 15.6%) | CAD (10, 11.1%) | .514 | |
| OSA (8, 8.9%) | OSA (16, 17.8%) | .122 | |
| Recurrent stroke | Yes (36, 40%) | Yes (32, 35.6%) | .653 |
| WST-A | 1 (4, 4.4%) | 1 (2, 2.2%) | .507 |
| 2 (6, 6.7%) | 2 (12, 13.3%) | ||
| 3 (2, 2.2%) | 3 (4, 4.4%) | ||
| 4 (18, 20.0%) | 4 (16, 17.8%) | ||
| 5 (60, 66.7%) | 5 (56, 62.2%) | ||
| Mouth-opening degree | 1 (68, 75.6%)Others (22, 24.4%) | 1 (56, 62.2%)Others (34, 37.8%) | .030∗ |
| DSS | 1 (60, 66.7%)Others (30, 33.3%) | 1 (48, 53.3%)Others (42, 46.7%) | .003∗ |
| D between onset to admission | 27.1 ± 22.6 | 32.2 ± 31.2 | .205 |
| Stroke type | Ischemic (74, 82.2%)Hemorrhage (16, 17.8%) | Ischemic (72, 80.0%)Hemorrhage (18, 20.0%) | .854 |
| Stroke site | Supratentorial (40, 44.4%)Infratentorial (50, 55.6%) | Supratentorial (40, 44.4%)Infratentorial (50, 55.6%) | 1 |
| Depression | Yes (4, 4.4%) | Yes (10, 11.1%) | .144 |
| Disorder of consciousness | Awake (84, 93.3%) | Awake (78, 86.7%) | .206 |
| Tracheotomy | Yes (12, 13.3%) | Yes (22, 24.4%) | .081 |
| MMSE | Normal (64, 71.1%)Abnormal (26, 28.9%) | Normal (46, 51.1%)Abnormal (44, 48.9%) | .006∗ |
| BI | 44.4 ± 36.6 | 28.5 ± 32.7 | .002∗ |
| BBS | 22.7 ± 23.4 | 12.6 ± 19.8 | .002∗ |
| HB (g/L) | 130.0 ± 18.0 | 128.2 ± 19.0 | .492 |
| ALB (g/L) | 38.5 ± 3.74 | 37.5 ± 5.19 | .121 |
| LOS | 38.7 ± 27.9 | 37.6 ± 27.3 | .779 |
Significant difference was considered as P < .05.
ALB = albumin, BBS = Berg balance scale, BI = Barthel index, BMI = body mass index, CAD = coronary artery disease, DM = diabetes mellitus, DSS = drooling severity scale, HB = hemoglobin, LOS = length of stay, MMSE = mini-mental state exam, OSA = obstructive sleep apnea, WST-A = WST level on admission.
3.2. Regression models
Logistic regression results (Table 2) reveled Type of stroke, MMSE degree, DSS and HB level shown significant predictive value for PSD outcome. In addition, OSA (OR = 4.39, P = .105), DSS degree (OR = 5.51, P = .005) were risk factors for PSD recovery.
Table 2.
Logistic regression of PSD recovered or unrecovered.
| Variables | OR | 95% CI | β | P |
| Gender | 0.88 | 0.26∼2.98 | –0.12 | .843 |
| Age | 0.99 | 0.93∼1.04 | –0.01 | .614 |
| BMI | 0.80 | 0.62∼1.00 | –0.22 | .065 |
| Smoking | 2.16 | 0.38∼12.90 | 0.77 | .382 |
| Drinking | 0.23 | 0.02∼2.08 | –1.45 | .204 |
| DM | 0.32 | 0.07∼1.31 | –1.15 | .121 |
| Hypertension | 2.48 | 0.84∼7.97 | 0.91 | .109 |
| CAD | 0.94 | 0.23∼4.02 | –0.06 | .936 |
| OSA | 4.39 | 0.79∼30.22 | 1.48 | .105 |
| Depression | 0.39 | 0.03∼6.22 | –0.93 | .484 |
| Recurrent stroke | 0.32 | 0.09∼1.00 | –1.15 | .060 |
| Type of stroke | 0.17 | 0.03∼0.92 | –1.76 | .050∗ |
| Stroke site | 0.97 | 0.34∼2.70 | –0.03 | .950 |
| MMSE | 0.10 | 0.01∼0.60 | –2.34 | .017∗ |
| BI | 0.99 | 0.93∼1.05 | –0.01 | .652 |
| BBS | 0.98 | 0.91∼1.06 | –0.02 | .651 |
| Mouth-opening degree | 1.05 | 0.26∼4.20 | 0.05 | .946 |
| DSS | 5.51 | 1.77∼19.29 | 1.71 | .005∗ |
| D between onset to admission | 1.01 | 0.99∼1.04 | 0.01 | .372 |
| Hb | 0.95 | 0.91∼0.99 | –0.05 | .020∗ |
| Alb | 0.99 | 0.83∼1.17 | –0.01 | .896 |
| Disorder of consciousness | 0.12 | 0.01∼2.32 | –2.10 | .158 |
| Tracheotomy | 1.19 | 0.13∼13.33 | 0.17 | .883 |
| R2 | 0.32 |
ALB = albumin, BBS = Berg Balance scale, BI = Barthel Index, BMI = Body Mass Index, CAD = coronary artery disease, CI = Confidence Interval, DM = diabetes mellitus, DSS = Drooling severity scale, HB = hemoglobin, MMSE = Mini-Mental State Exam, OR = Odds Ratio, OSA = Obstructive sleep apnea.
The results of Multilinear regression model were shown as Table 3. Gender, BMI, drinking, hypertension, recurrent stroke, WST level on admission, BBS, DSS and days between onset to admission have significant predictive value for LOS of PSD patients. Furthermore, female, drinking, hypertension, high BBS score are protcctive factors, higher BMI, recurrent stroke, higher WST level on admission, higher degree of DSS, longer days between onset to admission are risk factors. In addition, the results of 2 regression models were display as Figure 1.
Table 3.
Multilinear regression of LOS for PSD patients.
| variables | β | SE | P |
| Gender | –17.322 | 4.680 | .000∗ |
| Age | –0.399 | 0.210 | .060 |
| BMI | 2.145 | 0.842 | .012∗ |
| Smoking | –4.270 | 6.592 | .519 |
| Drinking | –18.830 | 8.079 | .022∗ |
| DM | 7.176 | 5.164 | .168 |
| Hypertension | –11.331 | 3.994 | .006∗ |
| CAD | 2.429 | 5.253 | .645 |
| OSA | –11.940 | 6.502 | .069 |
| Depression | 11.332 | 9.704 | .246 |
| Recurrent stroke | 10.285 | 4.156 | .015∗ |
| Type of stroke | 1.012 | 6.522 | .877 |
| Stroke site | 1.374 | 3.839 | .721 |
| WST-A | 7.546 | 2.189 | .001∗ |
| MMSE | 0.075 | 7.020 | .991 |
| BI | 0.415 | 0.215 | .056 |
| BBS | –1.127 | 0.287 | .000∗ |
| Mouth-opening degree | –5.942 | 5.459 | .279 |
| DSS | 15.269 | 4.161 | .000∗ |
| D between onset to admission | 0.526 | 0.104 | .000∗ |
| HB | –0.001 | 0.156 | .996 |
| ALB | 0.460 | 0.589 | .437 |
| Disorder of consciousness | –5.795 | 11.840 | .626 |
| Tracheotomy | 13.292 | 9.240 | .153 |
| R2 | 0.64 |
ALB = albumin, BBS = Berg balance scale, BI = Barthel index, BMI = body mass index, CAD = coronary artery disease, DM = diabetes mellitus, DSS = drooling severity scale, HB = hemoglobin, MMSE = mini-mental state exam, OSA = obstructive sleep apnea, SE = standard error, WST-A = WST level on admission.
Figure 1.
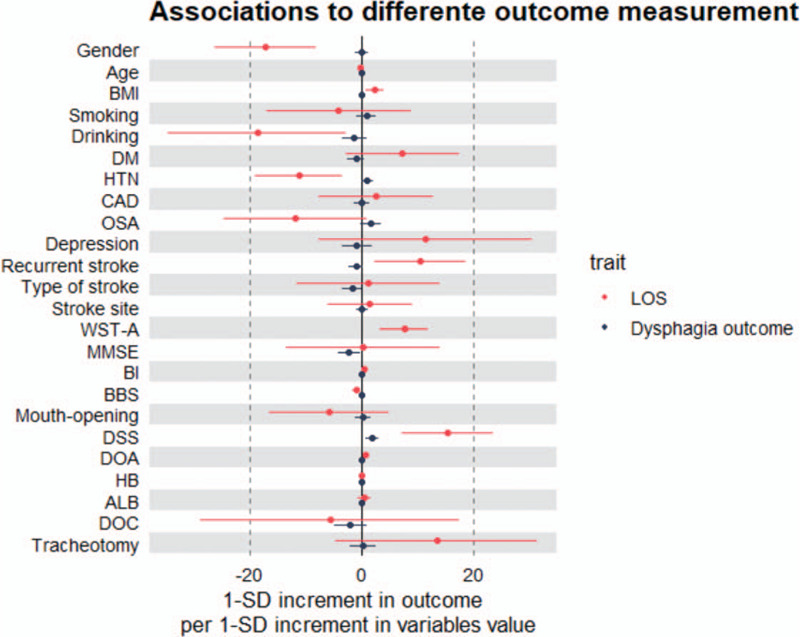
Difference of variables contribute to different outcome measurement of PSD. DOA = days between onset to admission, DOC = disorder of consciousness, LOS = length of stay, WST-A = WST level on admission.
4. Discussion
The current study focused on how the demographic and clinical variables affect the PSD outcome. However, overall, the logistic regression model is not a good model since the small value of R2, but the linear regression model performs better. Nevertheless, it can offer some insights for clinical treatment and further studies. The major reason caused the poor predictive value model is it is hard to measure the function during swallowing procedure.
Mouth-opening degree and DSS level measured on admission are parts of swallowing function. Therefore, it is easy to understand why significant differences were found between recovered and unrecovered groups. In addition, this study had investigated the relationship of cognitive function and dysphagia outcome, and concluded that the higher cognitive function remain on admission, the better PSD outcome may presence.[17] BI and BBS are measurements of disability, and this study reinforce the study from Raed A et al revealing severe disability was related to poor dysphagia outcome.[4]
It was highlighting that type of stroke, MMSE degree, HB and DSS degree shown significant predictive value for PSD recovery. The outcome of dysphagia after hemorrhagic stroke might be better than ischemic stroke, which is consistent with the study from Kumaresan et al revealing ischemic stroke are most likely have severe dysphagia.[18] This might can been attribute to the worsen overall condition of ischemic stroke patients since studies have demonstrated ischemic stroke shows high performance of diabetes, prior stroke, CAD.[18] Worsen condition on admission would contribute to a worsen outcome not only swallowing but also mobility and cognition.[19]
Interestingly, better MMSE degree was found in recovered group, but lower MMSE degree is a protective factor for PSD outcome which is controversial with present study.[17] This discrepancy might due to the limitation of the regression model itself which is based on the idealized version of real-world. In addition, isolated variables are used for regression-based methods to predict and analyze the independent variables and outcome for the casual connection. Thus, the regression model will be influenced by the collinearity of the variables which was concluded in analysis.[20]
HB level represents nutritional condition. Researchers have revealed PSD is closely associated with poor nutrition,[21] but the casual relationship is not clear. It might be dysphagia resulting malnutrition which is well-know. It also might be malnutrition leading to a high possibility of dysphagia, but it is not clear whether improving nutrition can resulting better swallowing outcome.[21]
It is worth noting that OSA is an important risk factor for PSD outcome. Some studies reported patients with OSA are more likely to have impaired swallowing reflex.[22] Valbuza et al investigated the differences of swallowing function between OSA patients and volunteers, revealed OSA patients presents subclinical symptoms of swallowing dysfunction and come up with the dysfunction might is caused by snore related neuromuscular injury.[23] The function of pharynx and upper airways for patients with OSA might be influenced and mechanoreceptor feedback of pharynx or respiratory muscles might act as an essential role in this process. Furthermore, study suggested oropharyngeal sensory impairment has association with an insensitive inhibitory modulating input of reflex and swallowing related central control.[23] Therefore, previous impairment might be an interpretation of worsen swallowing outcome for OSA patients with PSD.
Female, drinking, hypertension, high BBS score have been revealed act as protective factors for PSD patients. Female have been reported a decreased LOS before.[24] Rist et al reported stroke motor functional outcome is modest positively related with low alcohol consumption.[25] Although no literature was found metioning the relationship between drinking and dysphagia outcome, dyaphagia is a part of the sympotoms of stroke and LOS is depended on the overall condition of patients. Surprisely, hypertension performed as a protective factor for LOS of PSD patients. It might can be attribute to hypertension is a stronger predictive factor for hemorrhagic stroke,[26] which performed a better outcome than ischemic stroke generally. It is easy to understand that higher BBS is a protective factor since it is a measurement of patients’ condition on admission.
Higher BMI, recurrent stroke, higher WST level on admission, higher degree of DSS, longer days between onset to admission act as risk factors for LOS of PSD patients. Kroll et al reported that higher BMI is related to a increase risk of ischemic stroke but a decreased risk of hemorrhagic stroke[27] and hemorrhagic stroke might has a better outcome than ischemic stroke[17] as mentioned before. Recurrent stroke and longer days between onset to admission[28] has been demonstrated as predictive factors for worsen condition of stroke patients. In addition, higher WST level on admission and higher degree of DSS is measurements of swallowing function, so it is not hard to comprehend these 2 are risk factors for PSD patients.
5. Limitation
-
1.
The treatment for different patients with different condition of swallowing function is diverse, so the result might be affected by treatment, more subgroup studies should be investigated in the future.
-
2.
Swallowing functional measurements are not so details, because it is hard to measure the function during swallowing and compare among individuals.
-
3.
Stroke volume did not include in this study, which is an important factor to affect the LOS.
-
4.
Large studies will be helpful to confirm the OSA function for PSD recovery since the sample size of OSA patients is a little small.
-
5.
The results shown disorder of consciousness might decrease LOS, it seems a little strange, but it can be attributed to some patients with disorder of consciousness might choose homecare because of economic pressure.
6. Conclusion
Mouth-opening degree, DSS level, MMSE degree, BI and BBS had shown significant difference between the recovered and unrecovered groups. Furthermore, type of stroke, MMSE degree, DSS and HB level were found to be significant independent factors in the logistic regression for PSD outcome prediction. Gender, BMI, drinking, hypertension, recurrent stroke, WST level on admission, BBS, DSS and days between onset to admission have significant predictive value for LOS of PSD patients. In addition, OSA performs as a risk factor for PSD recovery.
Author contributions
Conceptualization: Xi Zeng, Xingna Zhao.
Data curation: Xiao Xi, Heping Li, Liugen Wang, Xiran Yin, Jing Zeng.
Formal analysis: Xiao Xi.
Funding acquisition: Xi Zeng.
Investigation: Xiao Xi, Jing Zeng, Yali zhai.
Methodology: Xiao Xi, Heping Li, Xiran Yin, Yunyun Song, Xi Zeng.
Project administration: Xi Zeng, Xingna Zhao.
Resources: Heping Li, Yunyun Song, Yali zhai.
Supervision: Liugen Wang, Xi Zeng, Xingna Zhao.
Validation: Liugen Wang, Xi Zeng.
Visualization: Xi Zeng.
Writing – original draft: Xiao Xi.
Writing – review & editing: Xi Zeng, Xingna Zhao.
Glossary
Abbreviations: BBS = Berg balance scale, BMI = body mass index, CAD = coronary artery disease, DSS = drooling severity scale, HB = hemoglobin, LOS = length of stay, MMSE = mini-mental state exam, OSA = obstructive sleep apnea, PSD = post-stroke dysphagia, WST = water swallow test.
References
- [1].Hankey GJ. Stroke. Lancet 2017;389:641–54. [DOI] [PubMed] [Google Scholar]
- [2].Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- [3].Lakshminarayan K, Tsai AW, Tong X, et al. Utility of dysphagia screening results in predicting poststroke pneumonia. Stroke 2010;41:2849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Joundi RA, Martino R, Saposnik G, et al. Predictors and outcomes of dysphagia screening after acute ischemic stroke. Stroke 2017;48:900–6. [DOI] [PubMed] [Google Scholar]
- [5].Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia 2001;16:7–18. [DOI] [PubMed] [Google Scholar]
- [6].Hinchey JA, Shephard T, Furie K, et al. Formal dysphagia screening protocols prevent pneumonia. Stroke 2005;36:1972–6. [DOI] [PubMed] [Google Scholar]
- [7].Cohen DL, Roffe C, Beavan J, et al. Post-stroke dysphagia: a review and design considerations for future trials. Int J Stroke 2016;11:399–411. [DOI] [PubMed] [Google Scholar]
- [8].O’Keeffe ST. Use of modified diets to prevent aspiration in oropharyngeal dysphagia: is current practice justified? BMC Geriatr 2018;18:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park BH, Seo JH, Ko MH. Effect of 45 degrees reclining sitting posture on swallowing in patients with dysphagia. Yonsei Med J 2013;54:1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Logemann JA, Rademaker A, Pauloski BR, et al. A randomized study comparing the shaker exercise with traditional therapy: a preliminary study. Dysphagia 2009;24:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sayaca C, Serel-Arslan S, Sayaca N, et al. Is the proprioceptive neuromuscular facilitation technique superior to Shaker exercises in swallowing rehabilitation? Eur Arch Otorhinolaryngol 2019;2:497–504. [DOI] [PubMed] [Google Scholar]
- [12].Suntrup S, Marian T, Schröder JB, et al. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: a randomized controlled trial. Intensive Care Med 2015;41:1629–37. [DOI] [PubMed] [Google Scholar]
- [13].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ohura T, Hase K, Nakayama T, et al. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol 2017;17:131.doi: 10.1186/s12874-017-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Megan K, Joseph S, Katelynn F, et al. Reliability and validity of the berg balance scale in the stroke population: a systematic review. Phys Occup Ther Geriatr 2019;7:196–222. [Google Scholar]
- [16].Passi D, Bhanot P, Kacker D, et al. Oral submucous fibrosis: newer proposed classification with critical updates in pathogenesis and management strategies. Natl J Maxillofac Surg 2017;8:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jo SY, Hwang JW, Pyun SB. Relationship between cognitive function and dysphagia after stroke. Ann Rehabil Med 2017;41:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kumaresan A, Jagatheesan Alagesan, Vijayaraghavan R. Determinants of dysphagia following stroke. Ethiop J Health Dev 2019;3:147–52. [Google Scholar]
- [19].Paolucci S, Antonucci G, Grasso MG, et al. Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: a matched comparison. Stroke 2003;34:2861–5. [DOI] [PubMed] [Google Scholar]
- [20].Zhang X, Yuan Z, Ji. Network or regression-based methods for disease discrimination: a comparison study. BMC Med Res Methodol 2016;16:100.doi: 10.1186/s12874-016-0207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].O’Neill PA. Swallowing and prevention of complications. Br Med Bull 2000;56:457–65. [DOI] [PubMed] [Google Scholar]
- [22].Teramoto S, Sudo E, Matsuse T, et al. Impaired swallowing reflex in patients with obstructive sleep apnea syndrome. Chest 1999;116:17–21. [DOI] [PubMed] [Google Scholar]
- [23].Valbuza JS, de Oliveira MM, Zancanella E, et al. Swallowing dysfunction related to obstructive sleep apnea: a nasal fibroscopy pilot study. Sleep Breath 2011;15:209–13. [DOI] [PubMed] [Google Scholar]
- [24].Chang KC, Tseng MC, Weng HH, et al. Prediction of length of stay of first-ever ischemic stroke. Stroke 2002;33:2670–4. [DOI] [PubMed] [Google Scholar]
- [25].Rist PM, Berger K, Buring JE, et al. Alcohol consumption and functional outcome after stroke in men. Stroke 2010;41:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Camilla IH, Lene R, David, et al. Predictors of ischemic and hemorrhagic strokes among people living with hiv: the d: a:d international prospective multicohort study. Eclinical Medicine 2019;8:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Modrego PJ, Pina MA, Fraj MM, et al. Type, causes, and prognosis of stroke recurrence in the province of Teruel, Spain. A 5-year analysis. Neurol Sci 2000;21:355–60. [DOI] [PubMed] [Google Scholar]
- [28].Wang H, Camicia M, Terdiman J. Time to inpatient rehabilitation hospital admission and functional outcomes of stroke patients. PM R 2011;3:296–304. [DOI] [PubMed] [Google Scholar]


