Supplemental Digital Content is available in the text
Keywords: creatine kinase, C-statistics, hypoalbuminemia, logistic regression analysis, outlier value, panic value, ROC analysis
Abstract
The risk factors associated with 72-hours mortality in patients with extremely high levels of random plasma glucose (RPG) remain unclear.
To explore the risk factors predictive of 72-hours mortality in patients with extremely high RPG under heterogenos pathophysiological conditions.
Retrospective, single-center, case-controlled cross-sectional study.
University teaching hospital.
Adults over age 18 were selected from the medical records of patients at the Saitama Medical Center, Japan, from 2004 to 2013.
Extremely high RPG (≥500 mg/dl).
Mortality at 72 hours following the RPG test, regardless of hospitalization or in an outpatient setting. Multivariate logistic regression analysis was performed with adjustment for age, sex, body mass index (BMI), and RPG level. The final prediction model was built using the logistic regression model with a higher C-statistic, specificity, and sensitivity.
A total of 351 patients with RPG ≥500 mg/dl were identified within the 10-year period. The 72-hours mortality rate was 16/351 (4.6%). The C-statistics of the 72-hours mortality prediction model with serum albumin (ALB) and creatine kinase (CK) was 0.856. The probability of 72-hours mortality was calculated as follows: 1/[1 + exp (−5.142 + 0.901log (CK) −1.087 (ALB) + 0.293 (presence (1) or absence (0) of metastatic solid tumor)]. The sensitivity and specificity of this model was 75.5%.
The independent risk factors associated with 72-hours mortality in patients with RPG ≥500 mg/dl are hypoalbuminemia, elevated CK, and presence of a metastatic solid tumour. Further research is needed to understand the mechanisms and possible interventions to prevent mortality associated with extremely high RPG.
1. Introduction
The normal range of random plasma glucose (RPG) is <200 mg/dl (<11.1 mmol/L). Diabetes can be diagnosed based on an RPG ≥200 mg/dl in the presence of classical diabetes symptoms. Hyperglycemia is common in patients with and without a history of diabetes.[1] A critical (panic) value is defined as a value that represents a pathophysiological state with extreme deviation from normal, in which the condition would become life-threatening without prompt action.[2] Meanwhile, extreme outlier values are statistically expressed as below the 0.5 to 1.0 percentile value, or above the 99.0 to 99.5 percentiles.[3] In some cases, extreme outlier values should be interpreted as panic values, i.e., abnormal values that may endanger life if immediate and appropriate action is not taken.[2]
In hospital settings, physicians may encounter patients with RPG levels that greatly exceed the normal range. The presence of hyperglycemia, particularly severe hyperglycemia, is related to a higher risk of complications, longer stay in intensive care unit, and higher mortality rates.[4] Previous studies have reported that hyperglycemia is a prognostic factor for ischemic stroke and transient ischemic attack,[5] acute myocardial infarction,[6] acute heart failure,[7] malignancies,[8] and in elderly patients with pneumonia.[9]
The threshold of an extremely high RPG (≥500 mg/dl; 27.8 mmol/L) is defined as panic data by the Japanese Society of Laboratory Medicine 2018 guidelines[10] as well as Grade 4 values of Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03.[11] According to the National Cancer Institute in the United States, an extremely high RPG indicates life-threatening consequences or the need for urgent intervention. In a study by Guo, the 90-day mortality in patients with blood glucose levels >500 mg/dl was associated with sepsis, renal impairment with electrolyte imbalance, and lower blood pressure.[12]
Several studies have identified predictors of 72-hours mortality for patients in intensive care units,[13] after emergency department visits,[14] and those with trauma.[15] Siregar et al showed that the 72-hours mortality prediction model performed well; this model includes comorbidities, history of diabetic ketoacidosis (DKA), level of consciousness, and lactate levels in patients with DKA at a general hospital in Indonesia.[16] In patients with DKA, Efstathiou et al reported that serum glucose ≥500 mg/dl was predictive of 72-hours mortality.[17] It is important to understand what risk factors could predict poor 72-hours mortality in patients with extremely high RPG to improve the earlier management in clinical settings. However, the 72-hours mortality prediction model for patients with an extremely high outlier value of RPG is unclear.
We hypothesized that the creation of a novel prediction model for the 72-hours outcome in patients with extremely high RPG (≥500 mg/dl) could assist physicians with earlier decision-making and providing optimized therapeutic and management options to improve survival. We anticipate an improvement in the quality of initial medical management in both primary and critical care settings as a result of implementing these changes. We aimed to elucidate risk variables to predict 72-hours mortality among patients with extremely high RPG under both heterogeneous pathological conditions and any medical care setting in a university teaching hospital.
2. Patients and methods
2.1. Ethics approval
The study protocol was designed to the tenets of the Declaration of Helsinki and was approved by the Institutional Clinical Ethnics Review Boards (IRB) of Saitama Medical Center, Jichi Medical University, Saitama, Japan (Clinical #10–79). The approval did not include the option for sharing data in public; therefore, we ensured that all data of the patient records were fully anonymized. The data do not include any potentially identifying or sensitive patient information. The data are available to interested researchers upon reasonable request to the corresponding author and will be provided once approved by the IRB. The need for informed consent was waived due to the retrospective nature of the study.
2.2. Study design and participant selection
This was a retrospective single-center case-controlled cross-sectional study. Participants aged >18 years were selected from medical records in the Saitama Medical Center, Japan. We identified a cohort of 664,277 patients who underwent the plasma glucose test in the hospital's clinical laboratory within a 10-year period from 2004 to 2013. The overall incidence of an extremely high RPG (≥500 mg/dl) was 0.0941%, and 625 patients were defined. This patient selection was carried out regardless of whether the patients were hospitalized or in an outpatient setting, and by only focusing on an extremely high RPG ≥500 mg/dl not restricted to patients in the Emergency Department. We excluded patient records that were metachronous duplicates of the same patient (only the highest RPG value from each of these patients was considered), patients with cardiopulmonary arrest at arrival, patients with unknown outcomes, and erroneous blood samplings from the intravenous infusion route. After applying the exclusion criteria, a sample of 351 patients was selected for use a derivation dataset (Dataset-A). Dataset-B, composed of 550 patients with 300≤ RPG < 500 mg/dl from the year of 2013, was used for validation of the model, by selecting in same manner as was performed for Dataset-A. A flow chart of the selected cohort is shown in Figure 1.
Figure 1.
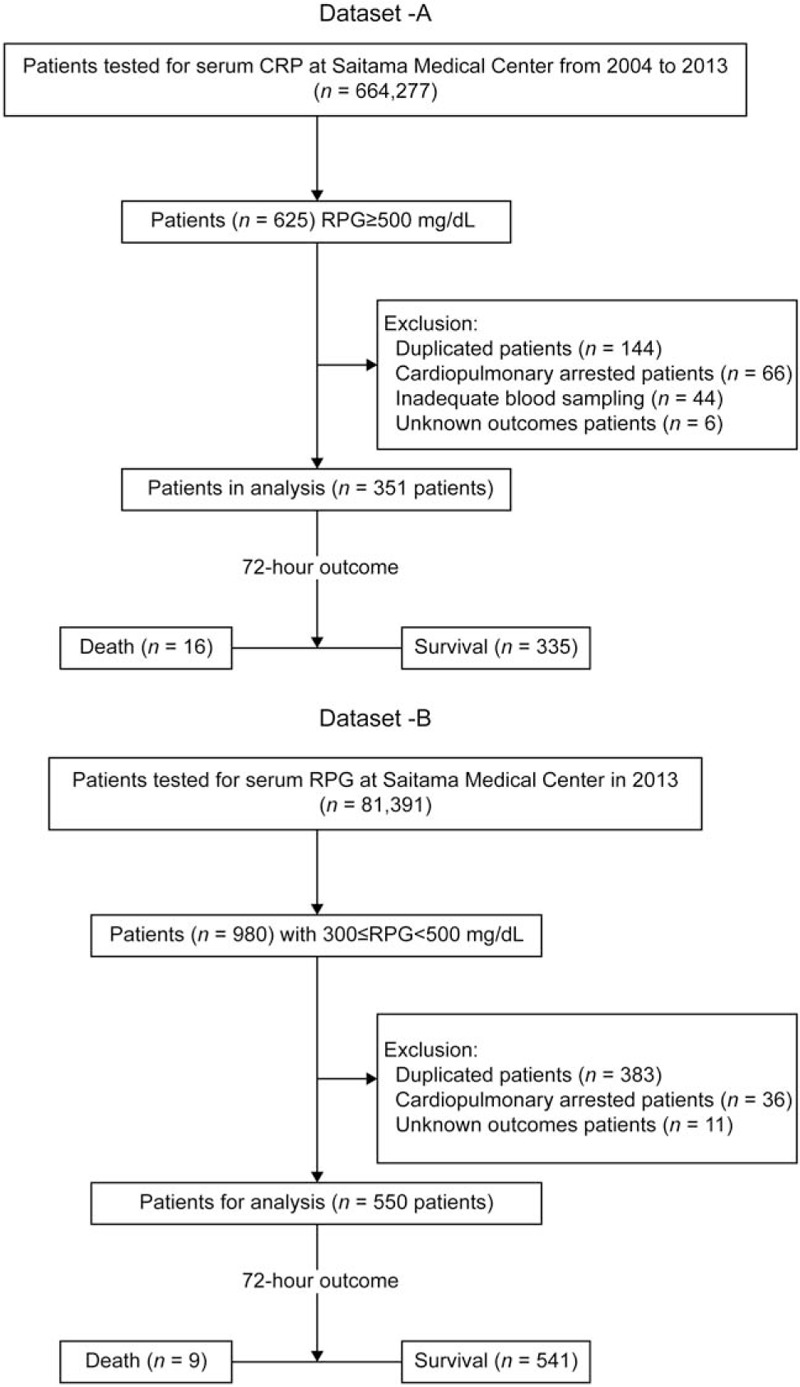
Flow diagram for patient selection.
The primary outcome was defined as 72-hours mortality following the RPG test. Cases were defined as patients with extremely high RPG levels who died during the first 72 hours after the test. Controls were patients with extremely high RPG levels who survived.
The following risk factors were tested for association with the 72-hours mortality outcome: age, sex, height, weight, body mass index (BMI), number of cigarettes smoked (Brinkman index), vital signs at the time of examination (systolic blood pressure [SBP], diastolic blood pressure [DBP], pulse rate [PR], respiratory rate [RR], and body temperature [BT]), laboratory test values (RPG, total protein [TP], albumin [ALB], total bilirubin [T-Bili], direct bilirubin [D-Bili], aspartate aminotransferase [AST], alanine aminotransferase [ALT], γ-glutamyl trans-peptidase [γ-GTP], lactate dehydrogenase [LDH], alkaline phosphatase [ALP], creatine kinase [CK], amylase, C-reactive protein [CRP], sodium [Na], potassium [K], chloride [Cl], calcium [Ca], phosphorus [P], blood urea nitrogen [BUN], creatinine [Cr], uric acid [UA], total cholesterol [TC], and triglyceride[TG]), comorbidities defined according to the updated Charlson Comorbidity Index (CCI),[18] and causes of extreme hyperglycemia.
2.3. Statistical analysis
2.3.1. Sample size
From a preliminary analysis, we obtained a deceased to survived ratio of 5:95 (mortality rate of 5%) among patients with RPG ≥500 mg/dl. Assuming a need for testing the utility of a binary risk variable by assessing the proportions of the 2 groups, the sample size required to detect a difference of 0.35 was calculated as 219 (10 for the deceased group vs. 209 for the survived group) by setting tails of 2, a power of 80%, and an alpha error of 5%.[19] Consequently, we expanded the actual data size to 351 (with expected data sizes of 16 vs 335 for the deceased and survived groups, respectively) to ensure attainment of a higher power.
2.3.2. Descriptive statistics
All continuous data were checked for the assumption of normality. All variables are presented as the median and the 2.5 to 97.5 percentiles. If there were insufficient data to calculate the 2.5 to 97.5 percentile, only median values were displayed. The group differences were tested using the Fisher exact test for nominal variables and the Mann–Whitney U test for continuous variables.
2.3.3. Association analysis
Variables with a skewed distribution were transformed for the regression analyses. For the laboratory tests, a distribution of values for each test was made approximately Gaussian by power transformation using the following Box-Cox formula: [20]
Where x and X are test results before and after the transformation, respectively. The powers used for the major laboratory tests were λ = 0.0 (log-transformation) for PRG, T-Bili, D-Bili, AST, ALT, γ-GTP, LDH, ALP, CK, amylase, CRP, and Na, λ = 0.3 for BUN, and λ = 0.7 for P, and TC.
The primary focus was to determine multiple risk factors associated with 72-hours mortality. First, the potential risk factors for 72-hours mortality were examined using univariate logistic regression analysis. The odds ratios (OR) and 95% confidence interval (CI) were estimated without adjustment (crude ORs). The C-statistics of covariates with a significant crude OR were determined by receiver-operating characteristic (ROC) analysis.
We next performed multivariable logistic regression analysis. We estimated the OR with 95% CI and adjusted for age, sex, BMI, and RPG level for all covariates that were significant in the univariate logistic regression analysis and the C-statistics by the ROC analysis. Clinically important risk factors that were significant in the multivariate logistic regression analysis were included in the final prediction model.
The prediction model was estimated using multivariate logistic regression analysis (MLRA), and a stepwise model was used to build the algorithm. Stepwise regression for all continuous data is a method of regressing multiple variables while simultaneously removing those that are not important so that the selected model includes the optimal combination of covariates of continuous data. In the final step, binary covariates, such as the CCI component and causes of extreme hyperglycemia, were added to the former selected model with continuous data and analyzed using MLRA with stepwise model to build an algorithm. In the prediction model, the logit function reflected the probability of death, defined as the 72-hours mortality.
Finally, the specificity of the regression model predicted by Dataset-A from patients with RPG ≥500 mg/dl was assessed by comparison with a model predicted independently by Dataset-B (550 records composed of 9 dead and 541 alive) from the patients with 300≤ RPG < 50 mg/dl.
The following statistics were estimated in multivariate logistic regression and ROC analyses: intercept, regression coefficient, χ2 value, P value, adjusted OR, 95% CI, C-statistics, sensitivity, and specificity. A P value <.05 was considered statistically significant. C-statistics were defined as the area under the curve (AUC) analyzed according to the ROC curve. In addition, we assessed the multicollinearity between covariates by the variance inflation factor (VIF). Multicollinearity was defined as positive when the VIF was ≥5.
2.3.4. Statistical software
The statistical package for the Stat Flex software version 7.0.11 (Artech Co. Ltd, Osaka, Japan) was used for data analysis. G∗Power version 3.1.9.4 (Germany) [19] was used for sample size calculation.
3. Results
3.1. Demographics of participants
Characteristics of the derivation group of Dataset-A are presented in Table 1. The 72-hours mortality rate was 16/351 (4.6%). The median age of the participants was 61 to 63 years, 65% to 69% were men, and the median BMI was 22 to 23 kg/m2. There were no differences in the RPG levels between groups. The RR and the levels of T-Bili, D-Bili, AST, ALT, CK, CRP, and Na were significantly higher in those who died, whereas the SBP, DBP, TP, ALB, and TG levels were significantly higher in the controls (Table 1).
Table 1.
Demographics of participants.
| 72-hour outcome | |||||
| Variables | Unit | n | Death (n = 16) Me (2.5–97.5 percentile) [n] | Survival (n = 335) Me (2.5–97.5 percentile) [n] | P-value |
| Age | years | 351 | 61.0 (44.0–78.0) [16] | 63.0 (28.0–86.1) [335] | .890 |
| Males, proportion | % | 237 | 68.8 [11] | 64.6 [226] | .914 |
| Height | cm | 331 | 165.0 (142.4–175.0) [15] | 162.0 (140.5–182.3) [316] | .564 |
| Weight | kg | 338 | 60.0 (34.8–90.0) [16] | 56.9 (35.8–88.9) [322] | .900 |
| BMI | kg/m2 | 329 | 22.9 (14.6–30.4) [15] | 21.9 (14.6–32.5) [314] | .967 |
| Brinkman index | cigarettes/day × years | 329 | 700 (0–940) [15] | 340 (0–2000) [314] | .209 |
| Vital Signs | |||||
| SBP | Mm Hg | 279 | 98.0 (62.0–141.0) [15] | 127.0 (67.8–221.8) [264] | .003 |
| DBP | Mm Hg | 277 | 59.0 (45.0–98.0) [15] | 74.0 (43.1–121.9) [262] | .018 |
| PR | beats/minute | 264 | 100.0 (36.0–140.0) [16] | 92.0 (53.7–142.2) [248] | .708 |
| RR | breath/minute | 126 | 30.0 (14.0–45.0) [11] | 20.0 (12.0–39.6) [115] | .028 |
| BT | °C | 206 | 36.80 (33.70–41.60) [15] | 36.70 (34.01–38.97) [191] | .555 |
| Biochemical examinations | |||||
| RPG | mg/dl | 351 | 626.5 (509.0–1655.0) [16] | 592.0 (502.9–1502.1) [335] | .482 |
| TP | g/dl | 320 | 5.70 (2.70–7.10) [15] | 6.90 (4.40–8.40) [305] | .0001 |
| ALB | g/dl | 320 | 2.70 (1.60–4.10) [15] | 3.60 (1.80–5.09) [305] | .0002 |
| T-Bili | mg/dl | 309 | 0.75 (0.25–4.36) [14] | 0.54 (0.18–2.89) [295] | .048 |
| D-Bili | mg/dl | 309 | 0.35 (0.13–3.46) [14] | 0.19 (0.06–1.54) [295] | .003 |
| AST | U/L | 333 | 35.5 (14.0–538.0) [14] | 23.0 (9.0–212.3) [319] | .015 |
| ALT | U/L | 333 | 38.0 (10.0–263.0) [14] | 25.0 (6.5–146.1) [319] | .017 |
| γ-GTP | U/L | 247 | 79.0 (8.0–2405.0) [12] | 84.0 (19.0–1089.8) [235] | .969 |
| LD | U/L | 285 | 136.0 (11.0–669.0) [12] | 81.0 (12.3–759.4) [273] | .276 |
| ALP | U/L | 305 | 282.5 (118.0–571.0) [14] | 359.0 (172.3–1324.7) [291] | .056 |
| CK | U/L | 308 | 355.0 (87.0–3359.0) [13] | 209.0 (33.8–955.4) [295] | .001 |
| Amylase | U/L | 179 | 153.0 (29.0–558.0) [8] | 66.0 (17.0–1187.8) [171] | .083 |
| CRP | mg/dl | 280 | 6.7 (0.2–20.0) [14] | 0.8 (0.0–29.6) [266] | .008 |
| Na | mEq/L | 311 | 135.0 (113.0–159.0) [15] | 131.0 (115.0–151.1) [296] | .032 |
| K | mEq/L | 311 | 4.50 (2.40–6.40) [15] | 4.65 (3.20–7.40) [296] | .760 |
| Cl | mEq/L | 311 | 102.0 (79.0–126.0) [15] | 96.5 (72.9–113.1) [296] | .098 |
| Ca | mg/dl | 228 | 8.55 (6.10–11.30) [12] | 8.80 (6.99–10.92) [216] | .418 |
| Corrected Ca | mg/dl | 220 | 9.75 (8.50–12.50) [12] | 9.60 (7.84–11.30) [208] | .320 |
| P | mg/dl | 226 | 5.20(0.70–10.90) [12] | 4.10 (2.00–11.24) [214] | .949 |
| BUN | mg/dl | 336 | 44.0 (14.0–184.0) [15] | 29.0 (9.0–109.9) [321] | .084 |
| Cr | mg/dl | 335 | 1.510 (0.480–3.130) [15] | 1.140 (0.420–8.960) [320] | .600 |
| UA | mg/dl | 265 | 6.95 (2.70–11.90) [10] | 6.30 (2.10–18.68) [255] | .545 |
| TC | mg/dl | 228 | 143.5 (92.0–274.0) [6] | 204.5 (96.1–454.9) [222] | .090 |
| TG | mg/dl | 226 | 94.0 (41.0–215.0) [5] | 188.0 (52.1–1227.1) [221] | .026 |
| Total updated CCI points | 351 | 3 (0–11) [16] | 3 (0–9) [335] | .086 | |
| Components of updated CCI (points) | n | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | |
| Congestive heart failure (2) | 351 | 7 (43.8) | 9 (56.2) | 76 (22.7) | 259 (77.3) | .069 |
| Dementia (2) | 351 | 0 (0.0) | 16 (100.0) | 32 (9.1) | 303 (90.9) | .379 |
| Chronic pulmonary disease (1) | 351 | 1 (6.3) | 15 (93.7) | 6 (1.8) | 329 (98.2) | .281 |
| Rheumatologic disease (1) | 351 | 0 (0.0) | 16 (100.0) | 13 (3.9) | 322 (96.1) | 1.000 |
| Mild liver disease (2) | 351 | 0 (0.0) | 16 (100.o) | 20 (6.0) | 315 (94.0) | .612 |
| Hemiplegia or paraplegia (2) | 351 | 0 (0.0) | 16 (100.0) | 26 (7.8) | 309 (92.2) | .619 |
| Mild to severe renal disease (1) | 351 | 0 (0.0) | 16 (100.0) | 52 (15.5) | 283 (84.5) | .143 |
| Diabetes with chronic complications or history of DKA (1) | 351 | 9 (56.3) | 7 (43.7) | 278 (83.0) | 57 (17.0) | .014 |
| Any malignancy, including leukemia and lymphoma (2) | 351 | 1 (6.3) | 15 (93.7) | 33 (9.9) | 302 (90.1) | 1.000 |
| Moderate or severe liver disease (4) | 351 | 1 (6.3) | 15 (93.7) | 25 (7.5) | 310 (92.5) | 1.000 |
| Metastatic solid tumour (6) | 351 | 7 (43.8) | 9 (56.2) | 30 (9.0) | 305 (91.0) | .001 |
| Acquired immunodeficiency syndrome/HIV infection (4) | 351 | 0 (0.0) | 16 (100.0) | 2 (0.6) | 333 (99.4) | 1.000 |
| Expected causes of extreme hyperglycemia | n | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | |
| Poorly controlled diabetes mellitus | 351 | 2 (12.5) | 14 (87.5) | 120 (35.8) | 215 (64.2) | .058 |
| Hyperglycemia emergency (DKA and HHS) | 351 | 2 (12.5) | 14 (87.5) | 86 (25.7) | 249 (74.3) | .376 |
| Infectious disease | 351 | 3 (18.8) | 13 (81.2) | 36 (10.7) | 299 (89.3) | .403 |
| Heart disease (ACS and AHF) | 351 | 3 (18.8) | 13 (81.2) | 24 (7.2) | 311 (92.8) | .116 |
| Steroid induced diabetes mellitus | 351 | 1 (6.3) | 15 (93.7) | 26 (7.8) | 309 (82.2) | 1.000 |
| Discontinuation of diabetes mellitus medication | 351 | 0 (0.0) | 16 (100.0) | 17 (5.1) | 318 (94.9) | 1.000 |
| Untreated diabetes mellitus | 351 | 0 (0.0) | 16 (100.0) | 17 (5.1) | 318 (94.9) | 1.000 |
| Cerebrovascular disease | 351 | 2 (12.5) | 14 (87.5) | 8 (2.4) | 327 (87.6) | 0.071 |
| Brain surgery | 351 | 1 (6.3) | 15 (93.7) | 1 (0.3) | 224 (99.7) | 0.089 |
| Others | 351 | 2 (12.5) | 14 (87.5) | 0 (0.0) | 335 (100.0) | 0.002 |
γ-GTP = γ-glutamyl transpeptidase, ACS = acute coronary syndrome, AHF = acute heart failure, ALB = albumin, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, Brinkman index = Number of cigarettes smoked, BT = body temperature, BUN = blood urea nitrogen, Ca = calcium, CCI = Updated Charlson Comorbidity Index, CK = creatine kinase, Cl = chloride, Cr = creatinine, CRP = C-reactive protein, D-Bili = direct bilirubin, DBP = Diastolic blood pressure, DKA = diabetic ketoacidosis, HHS = hyperosmolar hyperglycemic state, HIV = human immunodeficiency virus, K = potassium, LD = lactate dehydrogenase, Na = sodium, P = phosphorus, PR = pulse rate, RPG = random plasma glucose, RR = respiratory rate, SBP = systolic blood pressure, T-Bili = total bilirubin, TC = total cholesterol, TG = triglyceride, TP = total protein, UA = uric acid.
P values were calculated using Fisher exact test for categorical variables and the Mann–Whitney U test for continuous variables.
Regarding CCI components (Table 1), the proportion of metastatic solid tumours was significantly higher in cases than in controls (43.8% vs 9.0%, P = .001), whereas the proportion of patients with diabetes and chronic complications or history of DKA was significantly lower in cases than in controls (56.3% vs 83.0%, P = .014).
Regarding the expected causes of extreme hyperglycemia, a category of “other causes,” which included one case of ileus due to abdominal wall scarring hernia and another case of terminal gastric cancer, was found significantly more often in those who died than in those who survived (12.5% vs 0.0%, P = .027). The characteristics of the validation group of Dataset-B are presented in Supplementary Table S1, http://links.lww.com/MD/F597.
3.2. Univariate regression analysis
According to the crude (unadjusted) regression analysis, the significant risk factors associated with increased risk of the primary outcome were as follows: RR, metastatic solid tumour, CCI total score, cerebrovascular disease, brain surgery, D-Bili, AST, ALT, CK, CRP, Na, and Cl. In contrast, the risk factors that showed a protective effect were as follows: SBP, DBP, history of diabetes with chronic complications or DKA, TP, ALB, ALP, and TG (Table 2).
Table 2.
Univariate and multivariate logistic regression analysis results.
| Univariate logistic regression analysis | Multivariate logistic regression analysis | |||||
| Crude OR (95% CI) | P value | C-statistic | Adjusted OR (95% CI) by age, sex, BMI, and RPG | P value | C-statistic | |
| Vital signs | ||||||
| SBP (mm Hg) | 0.974 (0.956–0.994) | .0033 | 0.732 | 0.975 (0.956–0.994) | .0102 | 0.728 |
| DBP (mm Hg) | 0.968 (0.939–0.997) | .0304 | 0.681 | 0.968 (0.937–0.999) | .0448 | 0.683 |
| RR (breath/minute) | 10.55 (1.271–87.64) | .0291 | 0.700 | 10.80 (1.12–104.07) | .0395 | 0.743 |
| Updated Charlson comorbidity index | ||||||
| Diabetes with chronic complications or history of DKA | 0.264 (0.094–0.737) | .011 | 0.634 | 0.225 (0.070–0.728) | .0128 | 0.687 |
| Metastatic solid tumor | 7.907 (2.749–22.75) | .0001 | 0.674 | 10.17 (3.083–33.53) | .0001 | 0.712 |
| Total score | 1.208 (1.208–1.425) | .0246 | 0.625 | 1.269 (1.057–1.524) | .0108 | 0.663 |
| Expected causes of extremely hyperglycemia | ||||||
| Cerebrovascular disease | 5.839 (1.134–30.08) | .0349 | 0.551 | 7.011 (1.302–37.76) | .0234 | 0.606 |
| Brain surgery | 22.27 (1.328–373.44) | .0310 | 0.530 | 41.93 (1.561–1126.58) | .0261 | 0.543 |
| Biochemical examinations | ||||||
| TP (g/dl) | 0.415 (0.265–0.651) | .0001 | 0.806 | 0.415 (0.260–0.661) | .0002 | 0.795 |
| ALB (g/dl) | 0.300 (0.155–0.582) | .0004 | 0.790 | 0.255 (0.124–0.525) | .0002 | 0.815 |
| D-Bili (mg/dl) | 2.002 (1.184–3.383) | .0095 | 0.736 | 2.148 (1.220–3.783) | .0081 | 0.735 |
| AST (U/L) | 1.749 (1.103–2.773) | .0175 | 0.695 | 1.720 (1.050–2.817) | .0313 | 0.697 |
| ALT (U/L) | 2.020 (1.120–3.641) | .0194 | 0.690 | 2.027 (1.083–3.795) | .0272 | 0.680 |
| CK (U/L) | 2.276 (1.314–3.942) | .0033 | 0.768 | 3.142 (1.533–6.439) | .0176 | 0.779 |
| ALP (U/L) | 0.207 (0.052–0.817) | .0246 | 0.653 | 0.214 (0.051–0.902) | .0357 | 0.676 |
| CRP (mg/dl) | 1.503 (1.096–2.060) | .0114 | 0.712 | 1.602 (1.124–2.282) | .0091 | 0.742 |
| Na (mEq/L) | 1.815 (1.139–2.894) | .0122 | 0.664 | 2.199 (1.287–3.758) | .0039 | 0.685 |
| Cl (mEq/L) | 1.006 (1.002–1.011) | .0084 | 0.627 | 1.008 (1.003–1.013) | .0038 | 0.658 |
| TG (mg/dL) | 0.288 (0.092–0.904) | .033 | 0.791 | 0.112 (0.023–0.533) | .0060 | 0.941 |
ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CI = confidence interval, CK = creatine kinase, Cl = chloride, CRP = C-reactive protein, D-Bili = direct bilirubin, DBP = diastolic blood pressure, DKA = diabetic ketoacidosis, Na = sodium, OR = odds ratio, RPG = random plasma glucose, RR = respiratory rate, SBP = systolic blood pressure, TG = triglyceride, TP = total protein.
To calculate the odds ratio, the variations of sodium and chloride were 0.1, whereas the variations of others were 1.0.
3.3. Multivariate logistic regression analysis
When adjusted for age, sex, BMI, and RPG level, brain surgery was the strongest risk factor associated with an increased risk of 72-hours mortality. All other risk factors tested in the univariate model remained significant. In addition, the C-statistic of TG was the highest at 0.941 (Table 2).
3.4. Prediction model
Three covariates (ALB, CK, and the presence or absence of a metastatic solid tumour) were selected using stepwise regression analysis as the most significant predictors. The best combination of covariates to predict 72-h mortality with the highest C-statistic of 0.856 (95% CI = 0.775–0.937) was ALB plus CK, and the presence or absence of a metastatic solid tumour (Figure 2). The final prediction model coefficients were as follows:
| (1) |
Figure 2.

Comparison of the receiver-operating curves of predictive models for the 72-hour mortality of patients with extremely high outlier random plasma glucose values between Dataset-A and Dataset-B. The ROC was generated using Dataset-A for derivation and Dataset-B for validation. In order to test the specificity of the regression model for patients with RPG ≥ 500 mg/dl (Dataset-A), Dataset-B (patients with 300≤ RPG < 500 mg/dl) was applied to the following formula to compute the probability for fatal outcome. P = 1/[1 + exp (−5.142 + 0.901log (CK) - 1.087 (ALB) + 0.293 (metastatic solid tumour)]. The solid line shows the model of Dataset-A (C-statistics of 0.856 [95% CI = 0.775–0.937]), and the dotted line shows the ROC of Dataset-B (0.732 [95% CI = 0.535–0.929]).
Where “p” represents the probability for belonging to the deceased group to be calculated by assigning a set of variables from a given patient. This model predicted the 72-hours mortality of patients with extremely high RPG levels with 75.5% sensitivity and specificity (Table 3: Analysis 1).
Table 3.
Multivariate logistic regression analysis for predicting 72-hour mortality.
| Analysis (1) Dataset-A for derivation (RPG ≥500 mg/dl: 2004–2013) | |||||||
| MLRA: Obj Var = Death | n = 298 (with all 3 Exp Vars) | ||||||
| Exp Var | β | SE(β) | z | P | VIF | OR | 95% CI OR |
| −5.142 | 1.982 | ||||||
| ALB | −1.087 | 0.370 | −2.938 | 0.0033 | 1.035 | 0.337 | 0.163–0.696 |
| CK | 0.901 | 0.298 | 3.024 | 0.0025 | 1.009 | 2.463 | 1.373–−4.417 |
| Metastatic solid tumor | 0.292 | 0.118 | 2.484 | 0.0130 | 1.043 | 1.339 | 1.435–1.687 |
| AIC = 91.248, C-statistics = 0.856 (95%CI = 0.775–0.937), Sn = 75.7, Sp = 75.5 | |||||||
| P = 1/[1 + exp (−5.142 + 0.901log (CK)−1.087 (ALB) + 0.293 (metastatic soid tumour)] | |||||||
| Analysis (2) Dataset-B for validation (300 ≦RPG < 500 mg/dl: 2013) | |||||||
| ROC analysis to evaluate the accuracy of predicted probability (p) for fatal outcome | |||||||
| N dead | N alive | C-S | 95% CI of C-S | n = 315 (with all 3 Exp Vars) | |||
| 9 | 541 | 0.732 | 0.535∼0.929 | ||||
| Analysis (2) Dataset-B for testing specificity (300≦ RPG < 500 mg/dl: 2013) | |||||||
| MLRA: Obj Var = Death | n = 476 (with all one Exp Var) | ||||||
| Exp Var | β | SE (β) | z | P | OR | 95% CI OR | |
| −14.700 | 2.839 | ||||||
| LD | 1.917 | 0.476 | 4.028 | 0.0001 | 6.801 | 2.676–17.285 | |
| AIC = 79.738, C-statistics = 0.848 (95%CI = 0.699–0.997), Sn = 83.8, Sp = 83.8 | |||||||
| P = 1/[1 + exp (−14.7 + 1.97log (LD)] | |||||||
β = Partial regression coefficient, ALB = albumin, CI = confidence interval, CK = creatine kinase, C-S = C-statistics, Exp Var = explanatory variable, LD = lactate dehydrogenase, MLRA = multivariate logistic regression analysis, Obj Var = object variable, OR = odds ratio, RPG = random plasma glucose, SE = standard error, Sn = sensitivity, Sp = specificity.
In order to test the specificity of the regression model for patients with RPG ≥500 mg/dl, the Dataset-B from patients with 300≤ RPG <500 mg/dl (n = 550; 9 dead, 541 alive) was also applied to Eq-1 to compute the probability for achieving a fatal outcome. The accuracy of the prediction was 0.732 [95% CI = 0.535–0.929] (Table 3: Analysis 2, Fig. 2). This reduction from 0.856 was not statistically significant since 0.856 is within the 95% CI of the C-statistics for Dataset-B. In addition, independent derivation of a mortality prediction model was performed using Dataset-B through stepwise selection of risk variables. The regression model was found to be different from Eq-1 as shown in Table 3: Analysis 3 in that only LD was newly included in the model with a C-statistic of 0.848 (95% CI = 0.699–0.997).
4. Discussion and conclusion
This is the first study to investigate the risk factors associated with 72-hours mortality in patients with severe hyperglycemia with heterogeneous pathological conditions and in any medical care setting in a university teaching hospital. The main findings were as follows: 1) The 72-hours mortality rate in patients with severe hyperglycemia was 4.6%, and 2) the main independent risk factors associated with 72-hours mortality were hypoalbuminemia, elevated CK, and the presence of a metastatic solid tumour.
The presence of concomitant life-threatening diseases is known to be associated with mortality to a greater extent than metabolic complications due to hyperglycemia or ketoacidosis. Indeed, we found that the presence of a metastatic solid tumour was strongly associated with the 72-hours mortality outcome. Generally, the presence or absence of a metastatic solid tumour is an infrequent diagnosis in Emergency Departments. In our university teaching hospital, we were able to collect information regarding “the presence or absence of a metastatic solid tumour” by searching the electronic medical records of past medical history and the referral forms from the previous hospitals or clinics. Thus, physicians should consider the outcome to be poor in patients with metastatic tumors with extremely high RPG.
Efstathiou et al demonstrated that the 6 independent predictors of DKA mortality were severe coexisting diseases, pH < 7.0 of blood gas analysis at presentation, regular insulin required >50 IU within the first 12 hours, serum glucose ≥500 mg/dl after 12 hours, depressed mental status, and fever after 24 hours.[17]Another retrospective study reported that 72-hours mortality in patients with DKA was closely associated with comorbidities, including CCI total score, history of DKA, levels of consciousness, and serum lactate.[16] Interestingly, in our study, diabetes with chronic complications or a history of DKA were protective risk factors for 72-hours mortality; this is likely because chronic hyperglycemia may function to abate adverse effects of acute hyperglycemia.[21]
ALB, the major serum protein, has multiple important physiological functions, and the serum ALB concentration closely correlates with health homeostasis. The normal serum concentration of ALB in healthy adults is approximately 3.5 to 5.0 g/L, and hypoalbuminemia reflects the nutritional status of the whole body. Pathological conditions that lower ALB, such as neoplasms and liver diseases, are known to increase mortality. The median serum ALB level in the death group was 2.7 g/dl; hence, the group was considered malnourished.[22] Many studies have established that hypoalbuminemia is one of the strongest prognostic factors related to mortality, morbidity, and length of hospitalization, including mortality in elderly patients, people with chronic obstructive pulmonary disease, heart failure, malignancies, and sepsis.[23–25] Therefore, the association between hypoalbuminemia and 72-hours mortality in patients with extremely high RPG levels found in our study has a biological explanation.
CK is an enzyme that catalyzes the reversible transformation of creatine and ATP to creatine phosphate plus ADP. CK plays an important role in the energy transduction of cells found in the skeletal muscle, heart, brain, and other tissues. Moreover, the serum CK level is a biomarker of the damage of CK-rich tissues, such as that found in acute myocardial infarction, lung cancer, acute kidney injury in patients with rhabdomyolysis, amyotrophic lateral sclerosis, and necrotizing fasciitis.[26,27] In an Asian population, the normal median (2.5–97.5 percentile) concentration of serum CK in healthy adults is 520 (222–818) U/L in men and 194 (163–225) U/L in nonpregnant women. In the current study, the median (2.5–97.5 percentile) of CK in the death group was 355.0 (87.0–3,359.0) U/L. Several studies have examined the relationship between the plasma glucose level and serum CK elevation caused by rhabdomyolysis in patients with DKA[28] and hyperosmolar nonketotic coma.[29] Among the mechanisms involved in the association between plasma glucose level and serum CK elevation, the imbalance of electrolytes, such as sodium and calcium caused by hyperglycemia-induced hyponatremia is thought to lead to rhabdomyolysis.[30] Moreover, it has been suggested that excessive interstitial edema may cause circulatory disturbance and ATP deficiency based on hypoxemia, which could lead to hyperpermeability of the cell membrane of muscle tissue and result in CK elevation.[31] In severe malaria with infection by Plasmodium vivax, retrospective analysis showed that the incidence of hypoalbuminemia and elevated serum CK were 73.6% and 50.9%, respectively.[32] This finding indicates that both hypoalbuminemia and elevated serum CK could be biomarkers for predicting the prognosis of severe disease. Although the exact pathogenesis may not be clearly understood, the results of this study on 72-hours mortality in patients with extremely high RPG levels associated with ALB plus CK levels are novel and require further investigation.
The risk factors identified in this study were relevant in predicting 72-hours mortality among patients with RPG ≥500 mg/dl under heterogenous pathophysiological conditions. Moreover, the regression model could be fitted to predict the 72-hours mortality among patients with 300≤ RPG < 500 mg/dl. Since the C-statistic of Dataset-A (0.856) was within the 95% CI (0.535–0.929) of the C-statistic of Dataset-B (0.732), we consider that the prediction formula was also applicable to the lower RPG group, although with a different set of explanatory variables (RPG ≥500 mg/dl vs 300≤ RPG < 500 mg/dl). As a result, we found that the selected parameters in the MLRA model changed appreciably from a set of ALB, CK, and metastatic solid tumour to a set of LDH (Table 3: Analysis 2 and 3). The possible reasons for this observation are that the risk factors associated with 72-hours mortality are quite different between Dataset-A (RPG ≥500 mg/dl) and Dataset-B (300≤ RPG < 500 mg/dl), or that it is theoretically not feasible to include all variables in building the regression model using MLRA.
Finally, based on this study, under both heterogeneous pathological conditions and any medical care setting, physicians should triage patients with RPG ≥500 mg/dl, especially in the case of concomitant high CK and hypoalbuminemia. We expect that the appropriate therapeutic interventions will improve outcomes.
4.1. Limitations
There are several limitations to this study. First, this was a single-center retrospective study conducted at Saitama Medical Center in Japan. Therefore, these findings may not be generalizable to other populations. Second, we selected patients with RPG ≥500 mg/dl, which constituted only 0.0941% of our cohort; therefore the clinical relevance of our findings for the general population is limited. In fact, the risk factors for 72-hours mortality identified by MLRA were different in patients with lower RPG levels. Finally, the sample size was assumed to detect a difference of 0.35 in proportion for a binary variable between risk factors and the outcome. Therefore, the possibility of false-positive results for risk factors with continuous variables with lower differences cannot be eliminated.
4.2. Conclusions and future research
This study found that the prognosis of patients with an extremely high level of RPG ≥500 mg/dl is associated with hypoalbuminemia, higher CK, and the presence of a metastatic solid tumour. Consequently, when treating patients with extremely high RPG levels, physicians can easily estimate the 72-hours mortality using the serum ALB plus CK levels, and by investigating the past and present history of the presence or absence of a metastatic solid tumour. These predictions are likely to improve the decision-making process.
It remains unknown why the 72-hours mortality of patients with an extremely high RPG level is associated with both lower ALB and higher CK. Further prospective studies are needed to validate the performance of the mortality prediction model in these patients, as well as to identify the mechanisms regarding how lower ALB and higher CK lead to mortality.
Acknowledgments
The summary of this study was presented at the 64th Annual Meeting of the Japanese Society of Laboratory Medicine (Kyoto, November 17, 2017). Editorial support, in the form of medical writing, assembling tables, and creating high-resolution images based on authors’ detailed directions, collating author comments, copyediting, fact checking, and referencing, was provided by Editage, Cactus Communications.
Author contributions
TM and HS contributed to the conceptualization, formal analysis, and acquisition of funding. TM also contributed to data curation, investigation, and writing the original manuscript draft. HS also supervised the study, contributed to the review and editing of the original manuscript, and revision of the manuscript. KS contributed to data curation of the study. AI, TF, and KO supervised the study. AI and TF contributed to the review and editing of the original manuscript. All authors gave approval to the final manuscript.
Conceptualization: Tamami Watanabe, Hitoshi Sugawara.
Data curation: Tamami Watanabe, Kai Saito.
Formal analysis: Tamami Watanabe, Hitoshi Sugawara.
Funding acquisition: Tamami Watanabe, Hitoshi Sugawara.
Investigation: Tamami Watanabe.
Supervision: Hitoshi Sugawara, Akira Ishii, Takahiko Fukuchi, Kiyoka Omoto.
Writing – original draft: Tamami Watanabe.
Writing – review & editing: Hitoshi Sugawara, Akira Ishii, Takahiko Fukuchi.
Glossary
Abbreviations: γ-GTP = γ-glutamyl transpeptidase, ALB = albumin, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUC = area under the curve, BMI = body mass index, Brinkman index = Number of cigarettes smoked, BUN = blood urea nitrogen, Ca = calcium, CCI = Charlson Comorbidity Index, CI = confidence interval, CK = creatine kinase, Cl = chloride, Cr = creatinine, CRP = C-reactive protein, CTCAE = common terminology criteria for adverse events, D-Bili = direct bilirubin, DBP = diastolic blood pressure, DKA = diabetic ketoacidosis, IRB = Institutional Review Board, K = potassium, LD = lactate dehydrogenase, Na = sodium, OR = odds ratios, P = phosphorus, ROC = Receiver Operating Characteristic, RPG = random plasma glucose, SBP = systolic blood pressure, T-Bili = total bilirubin, TC = total cholesterol, TG = triglyceride, TP = total protein, UA = uric acid, VIF = variance inflation factor.
References
- [1].Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med 2006;355:1903–11. [DOI] [PubMed] [Google Scholar]
- [2].Lundberg GD. Critical (panic) value notification: an established laboratory practice policy (parameter). JAMA 1990;263:709. [PubMed] [Google Scholar]
- [3].Nanasaki Y, Suwabe A. What is the panic value?: a discussion on a contemporary version of panic. J Japanese Soc Emerg Med 2017;20:489–98. [Google Scholar]
- [4].Leite SA, Locatelli SB, Niece SP, et al. Impact of hyperglycemia on morbidity and mortality, length of hospitalization and rates of re-hospitalization in a general hospital setting in Brazil. Diabetol Metab Syndr 2010;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pan Y, Cai X, Jing J, et al. Stress hyperglycemia and prognosis of minor ischemic stroke and transient ischemic attack: The CHANCE Study (Clopidogrel in high-risk patients with acute nondisabling cerebrovascular events). Stroke 2017;48:3006–11. [DOI] [PubMed] [Google Scholar]
- [6].Kim EJ, Jeong MH, Kim JH, et al. Clinical impact of admission hyperglycemia on in-hospital mortality in acute myocardial infarction patients. Int J Cardiol 2017;236:9–15. [DOI] [PubMed] [Google Scholar]
- [7].Targher G, Dauriz M, Tavazzi L, et al. Prognostic impact of in-hospital hyperglycemia in hospitalized patients with acute heart failure: Results of the IN-HF (Italian Network on Heart Failure) Outcome registry. Int J Cardiol 2016;203:587–93. [DOI] [PubMed] [Google Scholar]
- [8].Debata A, Yoshida K, Ujifuku K, et al. Hyperglycemia is associated with poor survival in primary central nervous system lymphoma patients. Tumouri 2017;103:272–8. [DOI] [PubMed] [Google Scholar]
- [9].Akirov A, Shimon I. The prognostic significance of admission blood glucose levels in elderly patients with pneumonia (GAP Study). J Diabetes Complications 2016;30:845–51. [DOI] [PubMed] [Google Scholar]
- [10].Japanese Society of Laboratory Medicine Editrial Board. Panic data. In: Japanese Society of Laboratory Medicine Guideline 2018. 2018th ed. Tokyo: UCHUDO YAGI BOOKS; 2018. p. 464. [Google Scholar]
- [11]. National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03 [Internet]. National Institutes of Institute, National Cancer. 2010. p. 115. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Google Scholar]
- [12].Guo YW, Wu TE, Chen HS. Prognostic factors of mortality among patients with severe hyperglycemia. Am J Manag Care 2015;21:e9–22. [PubMed] [Google Scholar]
- [13].Lemeshow S, Klar J, Teres D, et al. Mortality probability models for patients in the intensive care unit for 48 or 72 hours: a prospective, multicenter study. Crit Care Med 1994;22:1351–8. [DOI] [PubMed] [Google Scholar]
- [14].Goulet H, Guerand V, Bloom B, et al. Unexpected death within 72 hours of emergency department visit: were those deaths preventable? Crit Care 2015;19:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stewart K, Garwe T, Oluborode B, et al. Association of interfacility helicopter versus ground ambulance transport and in-hospital mortality among trauma patients. Prehosp Emerg Care 2020;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Siregar NN, Soewondo P, Subekti I, et al. Seventy-two hour mortality prediction model in patients with diabetic ketoacidosis: a retrospective cohort study. J Asean Fed Endocr Soc 2018;33:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Efstathiou SP, Tsiakou AG, Tsioulos DI, et al. A mortality prediction model in diabetic ketoacidosis. Clin Endocrinol (Oxf) 2002;57:595–601. [DOI] [PubMed] [Google Scholar]
- [18].Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- [19].Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G∗Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- [20].Ichihara K, Boyd JC. IFCC Committee on Reference Intervals and Decision Limits (C-RIDL). An appraisal of statistical procedures used in derivation of reference intervals. Clin Chem Lab Med 2010;48:1537–51. [DOI] [PubMed] [Google Scholar]
- [21].Fujino M, Ishihara M, Honda S, et al. Impact of acute and chronic hyperglycemia on in-hospital outcomes of patients with acute myocardial infarction. Am J Cardiol 2014;114:1789–93. [DOI] [PubMed] [Google Scholar]
- [22].Onal O, Ozgun G. Comparison of the course and prognosis of geriatric patients admitted to the intensive care unit according to BMI and albumin values. Anesthesiol pain Med 2016;6:e32509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jellinge ME, Henriksen DP, Hallas P, et al. Hypoalbuminemia is a strong predictor of 30-day all-cause mortality in acutely admitted medical patients: a prospective, observational, cohort study. PLoS One 2014;9:e105983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Corti MC, Guralnik JM, Salive ME, et al. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA 1994;272:1036–42. [PubMed] [Google Scholar]
- [25].Furukawa M, Kinoshita K, Yamaguchi J, et al. Sepsis patients with complication of hypoglycemia and hypoalbuminemia are an early and easy identification of high mortality risk. Intern Emerg Med 2019;14:539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Simpson JP, Taylor A, Sudhan N, et al. Rhabdomyolysis and acute kidney injury: creatine kinase as a prognostic marker and validation of the McMahon Score in a 10-year cohort: a retrospective observational evaluation. Eur J Anaesthesiol 2016;33:906–12. [DOI] [PubMed] [Google Scholar]
- [27].Liu L, He Y, Ge G, et al. Lactate dehydrogenase and creatine kinase as poor prognostic factors in lung cancer: a retrospective observational study. PLoS One 2017;12:e0182168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].M-ler-Petersen J, Andersen PT, Hj-ne N, et al. Nontraumatic rhabdomyolysis during diabetic ketoacidosis. Diabetologia 1986;29:229–34. [DOI] [PubMed] [Google Scholar]
- [29].Schlepphorst E, Levin ME. Rhabdomyolysis associated with hyperosmolar nonketotic coma. Diabetes Care 1985;8:198–200. [DOI] [PubMed] [Google Scholar]
- [30].Kashiura M, Sugiyama K, Hamabe Y. Association between rapid serum sodium correction and rhabdomyolysis in water intoxication: a retrospective cohort study. J intensive care 2017;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cupisti A, Chisari C, Morelli E, et al. Abnormal increase of creatine kinase plasma levels following muscle exercise in nephrotic patients. Nephron 1998;80:204–7. [DOI] [PubMed] [Google Scholar]
- [32].Naha K, Dasari S, Prabhu M. Spectrum of complications associated with Plasmodium vivax infection in a tertiary hospital in South-Western India. Asian Pac J Trop Med 2012;5:79–82. [DOI] [PubMed] [Google Scholar]


