Abstract
We measured C24:0 and C26:0-carnitines in dried blood spots by flow injection analysis-tandem mass spectrometry method to evaluate whether they can be used as markers for newborn screening of X-linked Adrenoleukodystrophy (X-ALD). We found that C26:0-carnitine was 95.1% and 44.7% sensitive for identifying male X-ALD cases and heterozygous females, respectively. False negatives were found for C24:0-carnitine (11/82) and C26:0-carnitine (4/82). We conclude that C24:0 and C26:0-carnitines may not be reliable markers for X-ALD screening.
Keywords: C24:0-carnitine, C26:0-carnitine, C24:0-lysophosphatidylcholine, C26:0-lysophosphatidylcholine, Newborn screening, X-linked Adrenoleukodystrophy
Abbreviations: X-ALD, X-linked adrenoleukodystrophy; DBS, dried blood spot; VLCFA-LPC, very long-chain fatty acid-lysophosphatidylcholine; FIA-MS/MS, flow injection analysis- tandem mass spectrometry
Highlights
-
•
C24:0, C26:0-carnitines were measured in DBS of confirmed X-ALD cases
-
•
Sensitivity of C26:0-carnitine was 95.1% for X-ALD males, 44.7% for heterozygous females
-
•
False negatives were found for C24:0-carnitine (11/82) and C26:0-carnitine (4/82)
-
•
C24:0, C26:0-carnitines are not reliable markers for X-ALD screening
1. Introduction
X-linked Adrenoleukodystrophy (X-ALD), an inherited peroxisomal disorder, is characterized by elevation of very long-chain fatty acid (VLCFA, ≥C22) concentrations in blood and other tissues [[1], [2], [3]]. Currently, C26:0-lysophosphatidylcholine (C26:0-LPC), a derivative of C26:0 fatty acid, which is increased in X-ALD, is included in newborn screening (NBS) panels to screen for X-ALD [[1], [2], [3], [4], [5]]. Studies have also reported an elevation in very long-chain acylcarnitines (C24:0 and C26:0-carnitines) in X-ALD [1,[4], [5], [6]]. We aimed to evaluate if C24:0 and C26:0-carnitines could be used as reliable markers for screening of X-ALD.
2. Material and methods
2.1. Ethical approval
The study was approved by the institutional ethics committee of the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India. Informed consent and relevant assents were obtained from the participants before sample collection.
2.2. Sample collection, extraction and C24:0 and C26:0-carnitines analysis
We measured C24:0 and C26:0-carnitines in dried blood spot (DBS) samples collected from 82 genetically confirmed, symptomatic X-ALD patients (age range: 3–62 years), 38 heterozygous females/mothers of X-ALD patients (age range: 26–55 years) and 1257 putative normal controls (males = 905,females = 352), by the flow-injection analysis tandem mass spectrometry (FIA-MS/MS) method using NeoBase2 Non-derivatized MSMS kit (Perkin Elmer, Inc., Waltham, MA, U.S.A). In patients, DBS was obtained between 2 weeks to 44 months after the onset of symptoms, during diagnostic evaluation for X-ALD. Blood samples were collected by finger prick and stored at −80 °C in zip-lock pouches containing desiccants, until analysis.
For this analysis, 3.2 mm spots were punched from each DBS card into a 96-well plate, 125 μL of extraction solution containing isotope-labelled internal standards (IS) added into each well, sealed with an adhesive cover, and kept in a microplate shaker at 750 rpm for 30 min at 45 °C. From this, 100 μL of the extract/well was transferred to another 96-well plate and placed in the Acquity UPLC autosampler at 21 °C (Xevo TQD, Waters Corporation, Milford, MA, USA). Here, 10 μL/sample was injected with an isocratic flow of mobile phase (Neobase2 Non-derivatized MSMS solvents, Perkin Elmer, Inc., Waltham, MA, U.S.A) and analysed in the electrospray ionization (ESI) positive and multiple reaction monitoring (MRM) modes, with 120 °C source temperature, 65 V cone voltage, and 350 °C desolvation temperature, in 1.5 min. The internal standard of C26:0-carnitine (2H3—C26) and C26:0, C24:0-carnitine fragments were monitored at 543.5 > 85, 540.5 > 85, 512.5 > 85, respectively. Neolynx of MassLynx4.1.1 software (Waters Corporation, Milford, MA, USA) was used to calculate the concentrations of C24:0, C26:0-carnitines in μmol/L. Cut-offs were defined by plotting the receiver operating characteristic (ROC) curve, and using these cut-offs, the sensitivity and specificity of the analytes for identifying X-ALD cases were calculated. C26:0 and C24:0-LPC concentrations were also measured in all DBS samples by the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method [7]. SPSS statistical software version 17.0 (IBM Corporation, NY, USA) and GraphPad Prism 5.1(California, USA) were used for performing statistical analyses.
3. Results
3.1. Quality control
The % accuracy and % coefficient of variation (CV) for intra-day (N = 6) and inter-day (N = 20) precision of C26:0-carnitine was 102.9%, 1.4% and 5.1%, for Neobase2 kit low control and 104.4%, 1.7% and 3.8% for the high control, respectively.
3.2. Concentration of C24:0 and C26:0-carnitines and LPCs in male X-ALD patients
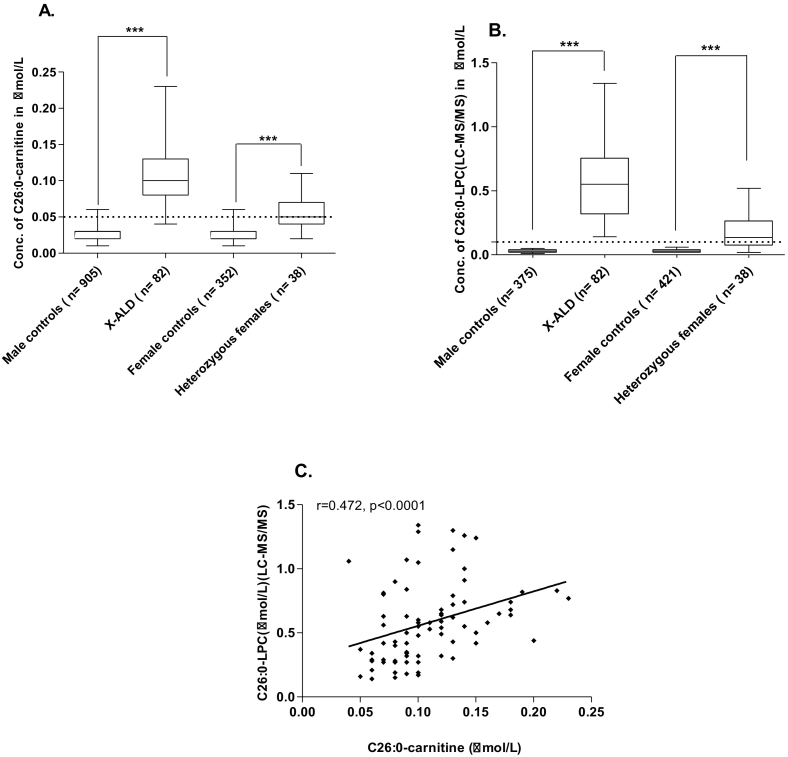
The median concentration (1st-99thpercentile) of C24:0 and C26:0-carnitines were 3.5 and 3.3-folds higher in X-ALD cases (n = 82, age range: 3–62 years) when compared to normal controls (males, n = 905, age range: 2–70 years), (cases - 0.07[0.02–0.22] μmol/L and 0.10[0.04–0.23] μmol/L vs. controls - 0.02[0.01–0.05] μmol/L and 0.03[0.01–0.06] μmol/L, respectively, Mann-Whitney U, P < 0.0001), (Fig. 1A). There was no age-dependant difference in the concentration of C24:0 and C26:0-carnitines in the controls.
Fig. 1.
(A) Box and whisker plots representing the median concentration (1st -99th percentile) of C26:0-carnitine in male controls, X-ALD patients, female controls and heterozygous females
(B) C26:0-LPC in male controls, X-ALD patients, female controls and heterozygous females.
(C) Spearman's correlation between C26:0-carnitine and C26:0-LPC in X-ALD patients.
Conc. – concentration; dotted lines represent the respective cut-off points for C26:0-carnitine and C26:0-LPC.
Receiver Operating Characteristic (ROC) cut-offs determined for C24:0, C26:0-carnitines were 0.04 and 0.05 μmol/L, respectively. Sensitivity, specificity and area under the curve (95% confidence interval, CI) was 86.6%, 97.3% and 0.97(0.96–0.99) respectively, for C24:0-carnitine and 95.1%, 98.7%, and 0.99(0.99–1.00) respectively, for C26:0-carnitine. False negatives were reported for C24:0-carnitine (11/82) and C26:0-carnitine (4/82). We did not find any association between the false negatives and the severity of the disease.
The above results were confirmed by the estimation of C24:0, C26:0-LPCs by the LC-MS/MS method [7], which was 100% sensitive and specific. The median concentration (1st-99thpercentile) of C24:0, C26:0-LPCs were 7, 18-folds higher in X-ALD cases (n = 82, age range: 3–62 years) when compared to normal controls (Males, n = 375, age range: 2–70 years), (cases - 0.56[0.20–1.48] μmol/L and 0.55[0.14–1.34] μmol/L vs. controls - 0.08[0.03–0.16] μmol/L and 0.03[0.01–0.05] μmol/L, respectively, Mann-Whitney U, P < 0.0001) (Fig. 1B).
A positive correlation (Spearman's r = 0.472, P < 0.0001) was observed between C26:0-LPC (LC-MS/MS) and C26:0-carnitine in X-ALD patients (Fig. 1C).
3.3. Concentration of C24:0 and C26:0-carnitines and C26:0-LPC in heterozygous females
Since C26:0-carnitine had a higher sensitivity among the two very long-chain acylcarnitine markers, we compared its median concentration in heterozygous females (n = 38, age range: 26–55 years) with female controls (n = 352, age range: 22–60 years), and found that C26:0-carnitine was 2.5 folds higher in cases in comparison to controls (0.05 [0.02–0.11] μmol/L vs. 0.02 [0.01–0.06] μmol/L, Mann-Whitney U, P < 0.0001) (Fig. 1A).
The sensitivity (95% CI) and specificity (95% CI) of C26:0-carnitine in identifying heterozygous females was 44.7% (28.6%–61.7%) and 98.3% (96.3%–99.3%). False positives (6/352) and false negatives (21/38) were reported for C26:0-carnitine measured by the FIA-MS/MS method.
The sensitivity (95% CI) and specificity (95% CI) of C26:0-LPC measured by the LC-MS/MS method was 65.7% (48.6%–80.3%) and 100% (99.1%–100%), respectively [7]. Blood C26:0-LPC was 4.3 folds higher in cases when compared to controls [0.13 (0.02–0.52) μmol/L vs. 0.03 (0.02–0.06) μmol/L, Mann-Whitney U, P < 0.0001] (Fig. 1B).
4. Discussion
With the addition of very long-chain acylcarnitines and VLCFA-LPCs to the newborn screening panels offered by commercially available kits, early diagnosis of X-ALD is feasible [3,6]. Newborns with X-ALD are asymptomatic. Due to the lack of newborn screening for X-ALD in India, cases are identified only during diagnostic evaluation for symptoms. The onset of symptoms varies from 3 years to over 60 years of age in males and typically over 60 years in females [2,3]. Our cohort represented individuals belonging to various regions and ethnic groups of the Indian population, with most of our patients belonging to South India (n = 36, 43.9%). The most predominant clinical phenotype in our cohort was the childhood cerebral form of X-ALD (n = 54, 65.85%) followed by the adolescent cerebral (n = 11, 13.4%) and adult cerebral forms (n = 9, 10.9%), Addison-only (n = 5, 6.09%) and adrenomyeloneuropathy (n = 3, 3.65%). Only one female carrier was symptomatic.
Tian and co-workers [6] have reported that C26:0, C24:0-carnitines and C26:0-LPC are important biomarkers for screening for X-ALD among children. In contrast, Huffnagel et al. [4] and Klouwer et al. [5] demonstrated that C26:0-LPC is a superior and sensitive marker for identifying VLCFA accumulation in newborns, adult males, and females with ALD in comparison to C26:0-carnitine. In our study on a relatively larger cohort conducted for the first time in the ethnically and culturally diverse Indian population, we found that C26:0-carnitine, measured by the FIA-MS/MS method, had a sensitivity of only 95.1% for identifying male X-ALD cases and 44.7% for identifying heterozygous females, compared to C26:0-LPC measured by LC-MS/MS, which was 100% and 65.7% sensitive in identifying males X-ALD cases and heterozygous females, respectively. False negative screening tests will eventually result in missing out the identification of X-ALD cases and can present an immense challenge to biomedical professionals involved in public health programmes, and the use of specific markers with a high degree of sensitivity that can reliably identify all affected subjects is vital for the success of such screening programmes.
5. Conclusion
We conclude that C24:0 and C26:0-carnitines are less reliable than C24:0, C26:0-LPCs for the biochemical screening of X-ALD.
Ethical approval
The study protocol was reviewed and approved by the Institutional Ethics Committee of the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India.
Funding
This research was funded by the Department of Biotechnology, Ministry of Science and Technology, New Delhi, Government of India .
Declaration of Competing Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgment
This work was supported by the Department of Biotechnology (DBT), Ministry of Science and Technology, New Delhi, Government of India. We thank Centers for Disease Control and Prevention, Atlanta, GA, USA, for including us in the Newborn Screening and Quality Assurance Programme, and for providing us with quality control and proficiency testing samples.
References
- 1.De Biase I., Tortorelli S., Kratz L., Steinberg S.J., Cusmano-Ozog K., Braverman N. Laboratory diagnosis of disorders of peroxisomal biogenesis and function: a technical standard of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. 2020;22:686–697. doi: 10.1038/s41436-019-0713-9. [DOI] [PubMed] [Google Scholar]
- 2.Br T., T. C, F. A, M. Ab X-linked adrenoleukodystrophy: pathology, pathophysiology, diagnostic testing, newborn screening and therapies. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2020 doi: 10.1002/jdn.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barendsen R.W., Dijkstra I.M.E., Visser W.F., Alders M., Bliek J., Boelen A., Bouva M.J., van der Crabben S.N., Elsinghorst E., van Gorp A.G.M., Heijboer A.C., Jansen M., Jaspers Y.R.J., van Lenthe H., Metgod I., Mooij C.F., van der Sluijs E.H.C., van Trotsenburg A.S.P., Verschoof-Puite R.K., Vaz F.M., Waterham H.R., Wijburg F.A., Engelen M., Dekkers E., Kemp S. Adrenoleukodystrophy newborn screening in the Netherlands (SCAN Study): the X-factor. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huffnagel I.C., van de Beek M.-C., Showers A.L., Orsini J.J., Klouwer F.C.C., Dijkstra I.M.E., Schielen P.C., van Lenthe H., Wanders R.J.A., Vaz F.M., Morrissey M.A., Engelen M., Kemp S. Comparison of C26:0-carnitine and C26:0-lysophosphatidylcholine as diagnostic markers in dried blood spots from newborns and patients with adrenoleukodystrophy. Mol. Genet. Metab. 2017;122:209–215. doi: 10.1016/j.ymgme.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Klouwer F.C.C., Ferdinandusse S., van Lenthe H., Kulik W., Wanders R.J.A., Poll-The B.T., Waterham H.R., Vaz F.M. Evaluation of C26:0-lysophosphatidylcholine and C26:0-carnitine as diagnostic markers for Zellweger spectrum disorders. J. Inherit. Metab. Dis. 2017;40:875–881. doi: 10.1007/s10545-017-0064-0. [DOI] [PubMed] [Google Scholar]
- 6.Tian G.-L., Xu F., Jiang K., Wang Y.-M., Ji W., Zhuang Y.-P. Evaluation of a panel of very long-chain lysophosphatidylcholines and acylcarnitines for screening of X-linked adrenoleukodystrophy in China. Clin. Chim. Acta. 2020;503:157–162. doi: 10.1016/j.cca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Natarajan A., Christopher R., Netravathi M., Bhat M., Chandra S.R. Liquid chromatography-tandem mass spectrometry method for estimation of a panel of lysophosphatidylcholines in dried blood spots for screening of X-linked adrenoleukodystrophy. Clin. Chim. Acta. 2018;485:305–310. doi: 10.1016/j.cca.2018.07.007. [DOI] [PubMed] [Google Scholar]