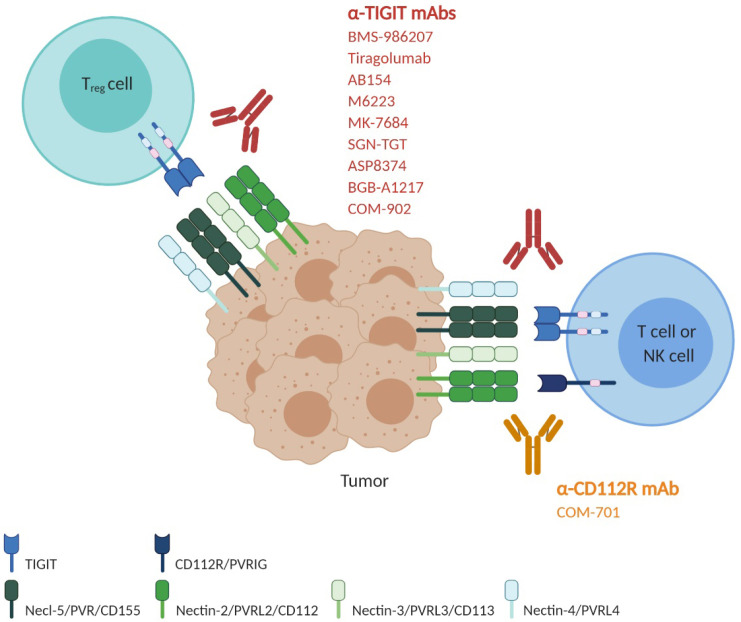
Fig. 2.
Blockade of the immune-checkpoint receptors TIGIT and CD112R using antagonistic monoclonal antibodies. Around nine human anti-TIGIT monoclonal antibodies, which have different IgG isotypes or mutant forms, have entered clinical trials. Given that combined blockade by TIGIT and PD-1/PD-L1 exhibited a more powerful antitumor effect in preclinical models, many clinical trials are evaluating the safety and efficacy of combination therapy with anti-TIGIT and anti-PD-1/PD-L1 as well as anti-TIGIT monotherapy. The most advanced, anti-TIGIT tiragolumab, is in a phase 3 trial in combination with the anti-PD-L1 atezolizumab and carboplatin and etoposide (CE) for treatment of untreated extensive-stage small-cell lung cancer (NCT04256421). Another phase 3 clinical trial is ongoing for the evaluation of tiragolumab combined with atezolizumab in patients with previously untreated locally advanced unresectable or metastatic PD-L1-selected NSCLC (NCT04294810). Phase I and phase II clinical trials using anti-TIGIT AB154, M6223, MK-7684, SGN-TGT, ASP8374, BGB-A1217 and COM-902, alone or combined with anti-PD-1/PD-L1 antibodies, are ongoing to evaluate the safety and efficacy in patients with metastatic or locally advanced solid tumors (NCT03628677, NCT04262856, NCT04457778, NCT02964013, NCT04165070, NCT04305054, NCT04305041, NCT04303169, NCT04254107, NCT03260322, NCT04047862 and NCT04354246). A phase I/II clinical trial with anti-TIGIT BMS986207, alone or combined with pomalidomide and dexamethasone, is ongoing in patients with relapsed refractory multiple myeloma who have relapsed after treatment with prior therapies (NCT04150965). There is one anti-CD112R antibody in a clinical trial. COM701 is a humanized anti-CD112R hinge-stabilized IgG4 developed by Compugen. COM701 is being tested in phase 1 clinical trials as monotherapy or in combination with nivolumab or in combination with BMS986207 and nivolumab, in patients with advanced solid tumors, including non-small cell lung cancer, ovarian, breast and endometrial cancer (NCT03667716 and NCT04570839).