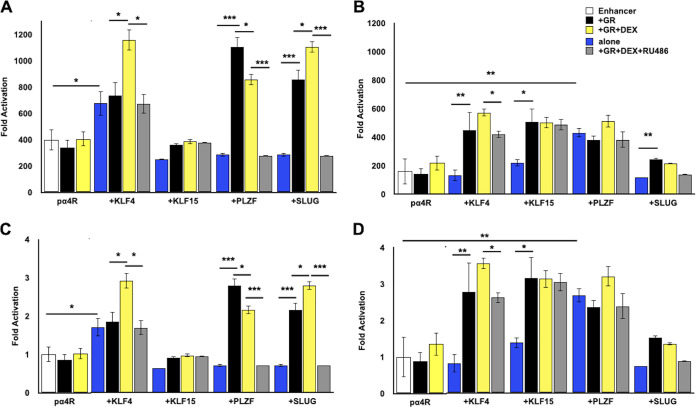
FIG 2.
Transactivation of ICP4 enhancer element by GR and stress-induced transcription factors. (A) Neuro-2A cells were grown in MEM containing 2% charcoal-stripped FBS and transfected with the pα4R construct (0.5 μg DNA), Renilla luciferase expression plasmid (0.05 μg DNA), GR expression plasmid (1 μg DNA), and, where denoted, KLF4, KLF15, PLZF, or SLUG (0.5 μg DNA). To maintain the same amount of DNA in each sample, empty vector was included in certain samples. At 24 h after transfection, designated cultures were treated with DEX (10 μM) or DEX and RU486 (10 μM each). (B) Vero cells were transfected with the denoted plasmids as described in the legend to panel A. Cells were harvested at 48 h after transfection, and protein lysate was subjected to dual-luciferase assay as described in Materials and Methods. Promoter activity was calculated as firefly luciferase activity compared to the transfection control, Renilla luciferase. For panels A and B, fold activation is presented as fold increase in promoter activity versus the empty minimal promoter reporter plasmid, pGL4.24[luc2/minP]. For panels C (Neuro-2A cells) and D (Vero cells), fold activation was presented as the fold increase in pα4R activity when cotransfected with the designated expression constructs relative to basal levels of pα4R promoter activity. The results are the mean from three separate experiments. Asterisks denote significant differences between the indicated treatments (*, >0.05; **, >0.01; ***, >0.001). Student’s t test was performed to determine significance.