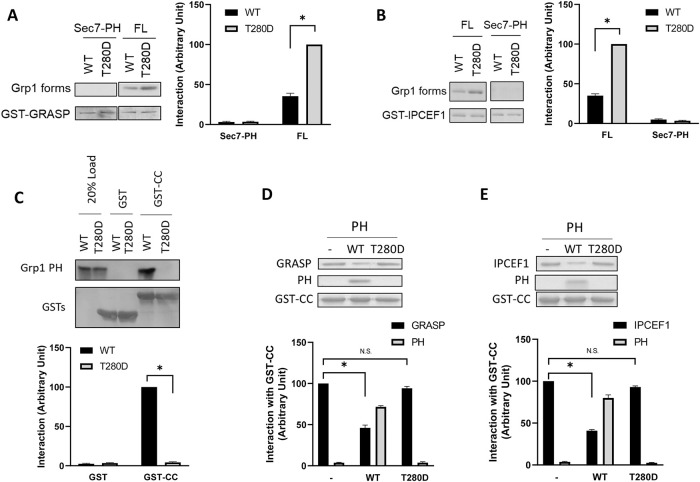
FIGURE 5:
T280 phosphorylation enhances the interaction between the CC domain of Grp1 and GRASP/IPCEF1. Quantitative results are shown as mean with standard error: *, p < 0.05, NS p > 0.05, Student’s t test. The number of independent experiments performed is specified below. (A) GRASP can interact directly with Grp1, which requires the CC domain in Grp1, and is modulated by the T280 phosphorylation. GRASP as a GST fusion protein was bound to beads and then incubated with various Grp1 constructs as indicated in pull-down experiments. A representative result along with quantitation from three experiments is shown. (B) IPCEF1 can interact directly with Grp1, which requires the CC domain in Grp1, and is modulated by the T280 phosphorylation. IPCEF1 as a GST fusion protein was bound to beads and then incubated with various Grp1 constructs as indicated in pull-down experiments. A representative result along with quantitation from three experiments is shown. (C) Reconstituting the intramolecular interaction between the CC domain and the PH domain in Grp1, and its release by the T280 phosphorylation. The CC domain as a GST fusion protein was bound to beads and then incubated with different forms of the PH domain as indicated in pull-down experiments. As negative control, a similar experiment was performed using GST alone. A representative result along with quantitation from three experiments is shown. (D) The direct interaction between the CC domain of Grp1 and GRASP is inhibited when the CC domain is preincubated with the wild-type, but not the T280D, form of the PH domain. The CC domain as a GST fusion protein was bound to beads and then incubated with GRASP in a pull-down experiment (left lane). The CC domain on beads was preincubated with the wild-type form of the PH domain, and then incubated with GRASP in a pull-down experiment (middle lane). The CC domain on beads was preincubated with the T280D form of the PH domain, and then incubated with GRASP in a pull-down experiment (right lane). A representative result along with quantitation from four experiments is shown. (E) The direct interaction between the CC domain of Grp1 and IPCEF1 is inhibited when the CC domain is preincubated with the wild-type, but not the T280D, form of the PH domain. The CC domain as a GST fusion protein was bound to beads and then incubated with IPCEF1 in a pull-down experiment (left lane). The CC domain on beads was preincubated with the wild-type form of the PH domain, and then incubated with IPCEF1 in a pull-down experiment (middle lane). The CC domain on beads was preincubated with the T280D form of the PH domain, and then incubated with IPCEF1 in a pull-down experiment (right lane). A representative result along with quantitation from four experiments is shown.