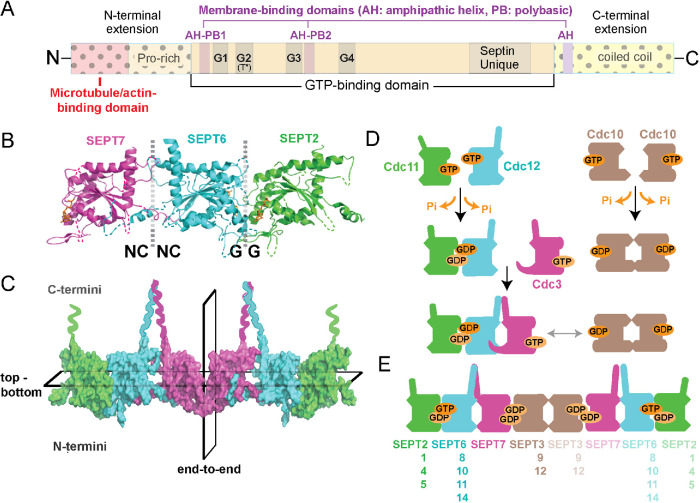
FIGURE 1:
Structure and assembly of septin GTPases. (A) Schematic shows the main domains of septin GTPases, which consist of a highly conserved GTP-binding domain with G1 (GxxxxGK[ST]), G3 (DxxG), and G4 (AKAD) motifs, and a septin unique element. Septins contain a catalytic threonine residue (T*), which corresponds to the Thr of the G2 motif of Ras GTPases and is absent from septin paralogues that lack GTPase activity. The N- and C-terminal extensions of the septin GTP-binding domain vary in sequence and length, and contain proline-rich and coiled-coil domains, respectively. Domains of cytoskeleton- and membrane-binding (amphipathic helices and polybasic sequences) are denoted and differ among septin paralogues. (B, C) The x-ray crystal structure of SEPT2/6/7 (PDB: 2qag; B) shows the NC-NC and G-G interfaces of dimerization, which alternate between the GTP-binding domains of consecutive septin subunits. The C- and N-terminal ends of the G-domains are located at the top and bottom of the dotted vertical lines, respectively, and the guanine nucleotide is depicted in orange. A surface representation of the SEPT2/6/7 heterohexamer (C) shows the symmetric (nonpolar) end-to-end arrangement of septin paralogues from the central homodimeric SEPT7 interface and the asymmetric positioning of the C- and N-terminal ends across the horizontal plane of multimerization. (D) Schematic of the assembly of yeast septin complexes, which is driven by successive events of homo- and heterodimerization that are determined by GTP binding and hydrolysis. (E) Schematic shows the subunit order and identity of mammalian septin hetero-octamers, and the interchangeability of paralogue subunits of the same septin group (Kinoshita rule).