Abstract
Background
Hearing impairment that is too severe to be adequately treated with conventional hearing aids can lead, in children, to severe developmental disturbances of hearing and language, and, in adults, to communicative and social deprivation. Recent advances in medical device technology and in microsurgical techniques have led to an expansion of the indications for cochlear implantation (CI) for adults with progressive hearing loss in older age, and to a restructuring of the process of care for these patients in Germany.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed, as well as on the CI guidelines and CI “white book” of the German Society of Otolaryngology and Head and Neck Surgery.
Results
Early and accurate diagnosis is crucial for the successful auditory rehabilitation of high-grade hearing impairment. In children, a key role is played by newborn auditory screening, which is mandatory in Germany and enables the provision of a CI in the first year of life when necessary. 86% of the children receiving a CI achieve linguistic comprehension of fluently spoken sentences. For adults, positive prognostic factors for hearing after the provision of a CI include a highly motivated patient, “postlingual” onset of the hearing impairment (i.e., after the acquisition of language), and a brief duration of deafness. Auditory rehabilitation is associated with significant improvement, not just of hearing and of the comprehension of spoken language, but also of quality of life, particularly in elderly patients. For patients of any age with bilateral hearing loss, CIs should be provided on both sides, if possible. The more common complications of the procedure, with a probability of 2–4% each, are technical implant defects, dizziness, and wound-healing disturbances.
Conclusion
Cochlear implantation, performed in specialized centers, is a safe and reliable technique and regularly enables the successful rehabilitation of hearing in both children and adults.
The data in the literature on the prevalence of hearing disorders in Germany in children and adults is scant and inconsistent. Two new systematic reviews have undertaken a detailed and critical analysis of this topic (1, 2). According to these reviews, hearing disorders in adults are reported to have a prevalence of 16–25%. There are currently no reliable data on the number adults with profound hearing impairment/deafness that could require cochlear implantation (1). The prevalence of hearing disorders in childhood and adolescence is 1–4%, with the prevalence of profound hearing loss (defined as deafness) being estimated at 0.01% (2).
Epidemiology.
Hearing disorders in adults are reported to have a prevalence of 16–25%. The prevalence of hearing disorders in childhood and adolescence is 1–4%, with the prevalence of profound hearing loss (defined as deafness) being estimated at 0.01%.
Effects of hearing loss.
Hearing forms the crucial basis of verbal communication and significantly contributes to social well-being.
The impact of impaired hearing, in particular profound hearing loss requiring cochlear implantation, is significantly underestimated in the population. In children, adequate hearing is an important prerequisite for age-appropriate social and language development (e1). Hearing forms the crucial basis of verbal communication and significantly contributes to social well-being. Hearing disorders can impair cognitive performance and promote social isolation, as well as the development of depressive and dementia-related disorders (3, 4). Particularly in elderly individuals, hearing impairments hamper spatial orientation and increase the risk of falls (e2).
Thus, cochlear implant (CI) treatment not only compensates hearing loss, but is of crucial importance to the health integrity, social participation, and increased quality of life of hearing-impaired individuals (5). The longer hearing loss remains untreated, the lower the likelihood of treatment success. Research is currently underway to determine the extent to which early auditory rehabilitation in elderly individuals with profound hearing loss can affect the course of neurodegenerative processes such as dementia.
Therefore, prompt diagnosis of hearing loss is of great importance at any age. Much like newborn screening, there are currently screening processes for older adults under development that could be routinely used by, e.g., by general practitioners or geriatric specialists (6).
General aspects of cochlear implant treatment
A cochlear implant is an electronic inner ear prosthetic device with an electrode array that is surgically inserted in the cochlea. In the case of inner ear damage, the intact auditory nerve is electrically stimulated and an auditory impression transmitted to the brain (Figure 1). The indication is made on the basis of a thorough ear, nose, and throat (ENT) and interdisciplinary pre-diagnostic work-up, which takes into consideration the patient‘s audiological, anatomical, and psychosocial conditions and is carried out in specialized centers (1, 7, 8).
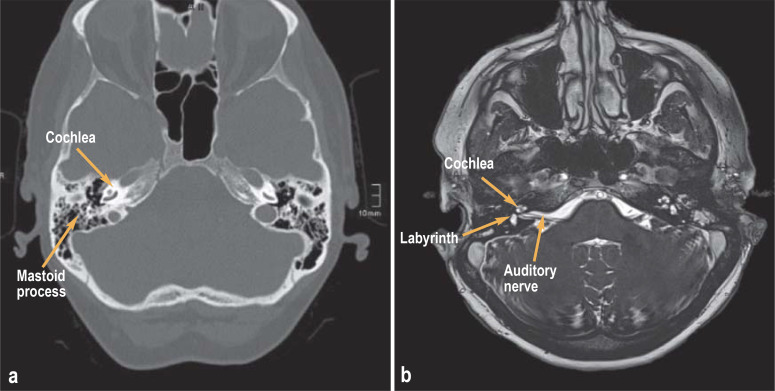
Figure 1:
Diagnostic imaging prior to cochlea implantation
a) Thin-slice petrous temporal bone computed tomography and
b) magnetic resonance imaging
The process of CI treatment includes implantation and the subsequent auditory rehabilitation phase, followed by lifelong annual follow-up care. The entire CI treatment process is set out in the CI White Book (7) and the CI guideline (8) and is the lifelong responsibility of the implanting center.
Today, the surgical techniques of cochlear implantation are considered to have few complications and are even possible in infants under the age of 12 months. A study of 219 children under the age of 3 years showed a low surgical complication rate (for example, wound infection) of 2% and an anesthesia complication rate (for example postoperative nausea) of 4.8% (9).
Learning objectives
Definition.
A cochlear implant is an electronic inner ear prosthetic device, the electrodes of which are surgically inserted in the cochlea and stimulate the auditory nerve.
The reader of this article should:
Gain an overview of the prevalence, incidence, and diagnosis of severe hearing impairments in children and adults
Become familiar with the different indications for cochlear implantation, the surgical principle, and the lifelong treatment process
Understand the importance of early cochlear implantation for auditory and speech rehabilitation as well as for the improvement of cognitive performance and quality of life.
Indications for CI treatment
Cochlear implantation is indicated (table 1) in profound hearing loss (bordering on deafness) that cannot be adequately treated through other auditory rehabilitation measures. One should expect to achieve better hearing and speech intelligibility with CI than with hearing aids (7). Therefore, before the decision for implantation is taken, appropriate treatment with (high-gain) air conduction hearing aids is required in the case of residual hearing that could potentially still be used for speech intelligibility. The definitive indication is then made by an ENT specialist on the basis of an extensive interdisciplinary clinical, audiological, radiological, and psychological diagnostic work-up.
Table 1. Indications for cochlear implantation.
| Hearing loss | CI treatment |
| Bilateral profound hearing loss (HL) bordering on deafness/deafness | Bilateral cochlear implantation (CI) |
| Unilateral HL with normal hearing in the contralateral ear | Unilateral cochlear implantation |
| Unilateral HL with impaired hearing in the contralateral ear | Bimodal treatment (CI + hearing aid in the contralateral ear) |
| Congenital bilateral HL | Bilateral CI within the first year of life |
| Acquired HL | Unilateral or bilateral CI with no age limit |
| HL with usable residual hearing in the low frequency range | Electric acoustic stimulation (EAS) CI + hearing aid on the affected side |
| HL following meningitis/labyrinthitis/trauma | Unilateral or bilateral CI preferably within 4–6 weeks |
CI. cochlear implant
Indication.
Cochlear implantation is indicated in profound hearing loss or hearing loss bordering on deafness that cannot be adequately treated by means of other auditory rehabilitation measures.
Due to the differing diagnostic possibilities in children and adults, a distinction is made between these two groups.
In children with congenital prelingual profound hearing loss, as well as perilingual deafness, the indication is primarily made on the basis of the audiological threshold, which is objectively determined using frequency-specific BERA (brainstem evoked response audiometry). The limit for CI indication today is considered to be an averaged audiological threshold of > 70 dB HL (10, 11), since it is generally no longer possible to achieve sufficient speech intelligibility with conventional hearing aids above this threshold. In addition to assessing the audiological threshold, one needs to take into consideration the level of language development, communication skills, general level of development, and socio-family aspects when determining indications as part of the interdisciplinary pre-diagnostic work-up (8). In the case of congenitally deaf children, implantation should be performed as early as possible in the first year of life once confirmatory diagnostics have been reliably completed (8, 12).
Limit for the indication of CI.
The limit for CI indication today is considered to be an average audiological threshold of >70 dB HL, since it is generally no longer possible to achieve sufficient speech intelligibility with conventional hearing aids above this threshold.
In children or adolescents and adults with postlingually acquired deafness, the assessment of maximum speech intelligibility in quiet at 65 dB sound pressure level (SPL), corresponding to the sound level of normal conversational speech, plays a central role in establishing the indication. In Germany, the Freiburg speech test is the standard method used to this end. When applied under free-field conditions, monosyllablic word intelligibility is evaluated unilaterally on each side. The indication limit for cochlear implantation is a maximum monosyllabic score with optimal hearing aid gain in quiet of 60% (7, 8). The following rule of thumb applies: if a patient can no longer make telephone calls with their hearing aid, the indication for CI should be reviewed.
In patients with postlingually acquired deafness, CI treatment should improve their monosyllabic score by at least 20 percentage points by the end of follow-up therapy, which is completed approximately 1 year after cochlear implantation in adults and after 3 years in children (7). In contrast to complete deafness, parameters that have a positive effect on the individually attainable speech intelligibility with CI include the presence of residual hearing that can still be measured preoperatively (13, 14) and a short duration of hearing loss in the ear to be implanted (15). However, the timing of CI treatment in adults with postlingual deafness is not limited by the time of onset of hearing loss, since successful implantation can still be achieved decades after deafness. On the other hand, the timing of cochlear implantation is critical in the case of deafness following meningitis, labyrinthitis, or trauma (for example, temporal bone fracture). Since there is a risk in such cases of early-onset inflammatory cochlear obliteration, which can prevent insertion of the CI electrode array, implantation should be performed as promptly as possible (4–6 weeks).
In individual selected cases of prelingually deaf adults, an indication for CI treatment can be considered if there is at least some level of receptive language ability (8, 16, 17).
With regard to audiological diagnosis and auditory rehabilitation planning, each ear needs to be evaluated and treated separately. In the case of bilateral profound hearing loss bordering on deafness, bilateral CI treatment is considered the standard today (18), and can be performed simultaneously or sequentially depending on the severity of hearing loss and the age of the patient. If the hearing loss is asymmetrical and one side still has adequate usable residual hearing with a hearing aid, bimodal treatment is performed in the form of ipsilateral CI treatment and a contralateral hearing aid.
Postlingually acquired hearing loss.
In patients with postlingually acquired deafness, CI treatment should improve their monosyllabic score by at least 20 percentage points by the end of follow-up therapy.
The maximum degree of asymmetrical hearing loss is unilateral deafness with normal contralateral hearing (19). Cochlear implantation is now also considered a desirable treatment for those patients with single-sided deafness wishing to receive detailed preoperative information, since this is the only way to ensure prompt rehabilitation of the damaged sensory organ. The treatment achieves significant improvements in sound localization and speech intelligibility in noise (20); it can also result in the suppression of tinnitus (21).
Diagnostic work-up prior to cochlear implantation
The preoperative diagnostic work-up helps establish the indication for cochlear implantation and verifies the feasibility of the surgical procedure, the subsequent basic and follow-up treatment, as well as aftercare, to restore hearing. Cochlear implantation is only indicated when all components are present.
The pre-diagnostic work-up involves an assessment of medical, medicotechnical, developmental psychological, and social indication criteria. These include: diagnosis of hearing and balance, an evaluation of operability, personalized implant selection, determining the surgical strategy and access route, and an assessment of the patient’s capacity for rehabilitation in their social environment.
Asymmetrical hearing loss.
The maximum degree of asymmetrical hearing loss is unilateral deafness with normal contralateral hearing.
The pre-diagnostic work-up requires interdisciplinary and interprofessional coordination between the various specialist disciplines (ENT medicine, neuroradiology, phoniatrics, and pedaudiology, neuropaediatrics, anesthesiology, and speech therapy, among others), as well as a consultation with the patient and all those in their environment. Children, adolescents, and adults require different consultation and treatment concepts.
Since the implanting center bears the responsibility for the process of lifelong CI treatment, this center needs to be involved in the pre-diagnostic work-up in all its complexity. Timely implantation is only possible if outpatient physicians and the centers providing CI treatment work closely together to establish the indication.
Pre-diagnostic work-up in adults
Adults represent the main indication group for CI treatment in Germany. These patients often have progressive age-related hearing loss that can no longer be adequately treated with a hearing aid; that is to say, it is no longer possible to achieve sufficient speech intelligibility for adequate everyday communication. In addition to taking a general patient history, one needs to establish the severity and duration of the hearing loss, as well as the patient‘s communication requirements in their social environment. This is followed by a complete ENT examination and comprehensive diagnosis of hearing and balance.
The pre-diagnostic work-up includes:
Diagnosis of hearing and balance, an evaluation of operability, personalized implant selection, determining the surgical strategy and access route, and an assessment of the patient‘s capacity for rehabilitation in his/her social environment.
The hearing diagnosis must confirm sensorineural hearing loss. Conductive hearing loss, hearing loss due to auditory neuropathy or the central auditory pathway do not generally represent indications for CI. One needs to compare an appropriate assessment of speech intelligibility using suitable hearing aids with the anticipated speech intelligibility following CI implantation. High-resolution computed tomography (CT) and magnetic resonance tomography (MRI) diagnosis of the petrous temporal bone are used to verify the presence of the cochlear nerve and the fluid filling the cochlea and to exclude chronic ear diseases or malformations (Figure 1). At the same time, this imaging also serves to aid surgical planning, involving determination of the access route and a personalized selection of electrodes based on the anatomical conditions. Today, depending on the electrodes selected, the length of electrodes required in the individual case can be determined on thin-slice CT of the petrous temporal bone using appropriate software. Since in some cases cochlear implants are not entirely MRI-compatible and produce an imaging artifact due to the magnets, follow-up diagnostics after implantation need to be coordinated with a neuroradiologist in the case of certain pre-existing conditions (e.g., cerebellopontine angle tumors, cholesteatoma). An important prerequisite of successful implantation is the patient’s capacity for rehabilitation, which requires relevant cognitive skills, manual skills to operate the audio processor, and appropriate social living conditions.
Contraindications for cochlear implantation.
Conductive hearing loss, hearing loss due to auditory neuropathy or the central auditory pathway do not generally represent indications for CI.
Finally, a pre-anesthetic assessment of the patient‘s fitness for anesthesia is performed. If fitness for general anesthesia is low or could involve risks, cochlear implantation can also be performed under local anesthesia in some cases.
Pre-diagnostic work-up in children
Profound hearing loss/deafness in childhood is often congenital or develops from progressive hearing loss in the first years of life. The mandatory neonatal screening for hearing impairment enables the earliest possible diagnosis in infants born deaf. Successful hearing and speech development is all the likelier the earlier implantation is performed (22).
In addition to a detailed patient history of hearing and speech development and subjective hearing tests adapted to the child‘s age, the child‘s pre-diagnostic work-up primarily focuses on objective hearing tests (auditory brainstem response test, otoacoustic emissions), as well as a determination of speech development and communication skills. Developmental psychological tests and an assessment of the socio-familial situation are also carried out. Using MRI and, if necessary, CT diagnosis, possible malformations of the inner ear and auditory nerve, as well as the choice of optimal electrode array, are assessed.
Surgical procedure for cochlear implantation
Cochlear implants are currently available as partially implantable hearing systems. As with hearing aids, the audio processor is worn behind the ear. A hearing loop that is magnetically attached to the scalp transmits the signal from the audio processor to the implant. The implant with the receiver is anchored beneath the skin in the temporal bone. The stimulation electrodes of the CI are inserted into the cochlea via the mastoid process and middle ear (Figure 1).
Pre-diagnostic work-up in children.
In addition to a detailed patient history of hearing and speech development and subjective hearing tests adapted to the child‘s age, the pre-diagnostic work-up in children focuses primarily on objective hearing tests, as well as a determination of speech development and communication skills.
Diagnostic imaging.
Using magnetic resonance imaging and computed tomography, possible malformations of the inner ear and auditory nerve, as well as the choice of suitable electrode array, are assessed.
Technique.
Cochlear implants are currently available as partially implantable hearing systems. As with hearing aids, the speech processor is worn behind the ear. A hearing loop that is magnetically attached to the scalp transmits the signal from the audio processor to the implant.
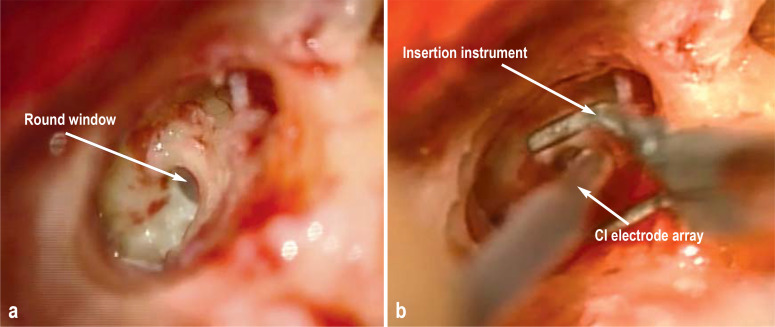
When introducing the electrode array into the cochlea, it is essential to spare important inner ear structures such as the basilar membrane and the organ of Corti. The atraumatic insertion of shorter electrodes via the round window helps preserve low-frequency residual hearing (23) (Figure 2). Cochlear implant patients with preserved low-frequency hearing (for example, patients with advanced age-related hearing loss) are able to achieve better hearing results, particularly in noise (24). These patients generally wear a combination of CI and hearing aid. The hearing aid acoustically stimulates at low frequencies, while the CI electrically stimulates the missing high frequencies (electric acoustic stimulation = EAS). This is indicated when the audiological threshold has not fallen more than 60 dB at frequencies up to 0.5 kHz (25).
Figure 2: Insertion of the cochlear implant (CI) electrodes.
a) Intraoperative visualization of the round window through the mastoid process and b) insertion of the CI electrode array in the scala tympani via the round window
If the anatomical conditions preclude low-risk insertion (for example, risk of damage to the facial nerve) via the round window, the electrode can alternatively be inserted via a cochleostomy. Here, the scala typmani is accessed via the promontory. The electrode array and the depth of insertion in the cochlea are selected on the basis of individual cochlear anatomy and residual hearing. Using imaging and electrophysiological techniques, it is already possible today to evaluate correct electrode placement (e3). Furthermore, each manufacturer offers a variety of different electrode arrays, making it possible to individually select electrodes in order to minimize trauma and optimize hearing success.
Auditory rehabilitation with cochlear implants
Successful auditory rehabilitation is defined as the patient‘s (re)gaining of hearing and speech ability. This involves a dynamic learning process over several months to years (26) and is divided into different stages of basic therapy, follow-up therapy and aftercare (7). Basic therapy (initial adjustment phase of the audio processor) is generally carried out by specialized professionals (CI audiologists) to set the individual electrical stimulation current intensity and stimulation parameters (26). The subsequent follow-up therapy (CI rehabilitation) is carried out using outpatient or inpatient interdisciplinary treatment concepts (teachers, speech therapists, audiologists, and physicians, among others) and is aimed at optimizing hearing and speech outcomes (27, 28). In a retrospective cohort study, postoperative CI auditory rehabilitation significantly increased the intelligibility of monosyllabic test words at a 65-dB presentation level (average 22.6%, SD 17.1%, N = 1 650; p < 0.001) ([27], Table 2). The lifelong aftercare that follows is aimed at ensuring long-term hearing and speech intelligibility as well as technical support with the implant. Auditory rehabilitation is accompanied by social and professional (re)integration measures.
Successful auditory rehabilitation.
Successful auditory rehabilitation is defined as the patient’s (re)gaining of hearing and speech ability. This is achieved through a dynamic learning process over several months to years.
As an interdisciplinary task, there are numerous special aspects of CI auditory rehabilitation in children. This is particularly the case since the possibility of “learning” to hear, understand speech, and speak are highly time-limited processes during child development and require particular attention (29).
Auditory rehabilitation results with CI in adults
Today, the goal of auditory rehabilitation with CI is speech intelligibility without the aid of lipreading, when telephoning, and in a noisy environment (background noise). Unilateral implants in adults achieve an average pre- to postoperative improvement of 44–65% in speech audiometry for monosyllabic test words (14, 30, e4) (table 2). In 96% of cases, CI achieves a better score in the Freiburg speech test (monosyllabic intelligibility at 65 dB without background noise) than would have been theoretically possible in the best case with hearing aids at maximum acoustic amplification (e5). Short duration of deafness, the onset of hearing loss after speech acquisition, and good residual hearing all represent factors that can make it possible to achieve significantly better speech intelligibility (31). Bilateral cochlear implantation shows significantly better results compared to unilateral CI implantation, with an 11% increase in the median speech intelligibility score in quiet (32).
Table 2. Details of the presented studies.
| Ref No. | Author/ year |
Study type | Number of studies (St)/ patient number (N) | Outcome parameters | Results |
| (27) | Zeh et al. 2015 | Retrospective SC cohort study | N = 1 650 | Change in MSI at 65 dB due to rehabilitation | Average increase 22.6 %. ± 17.1 %. min/max −25%/85%. p < 0.0001 |
| (30) | Boisvert et al. 2020 | Scoping review | Pre-CI: N = 1351/25 St Post-CI: N = 2798/46 St |
Change in MSI [%] pre-CI to post-CI | 8.2% to 53.9% |
| (e4) | Carlson 2020 | Retrospective SC cohort study | N = 259 | Change in MSI [%]_pre-CI to post-CI | 8% (IQR 0–24) to 58% (IQR 36–72) |
| (14) | Hoppe et al. 2019 | Retrospective SC case control study | N = 284. three groups after pre-CI MSI | Change in MSI [%] | Overall median increase: 65% |
| (32) | Blamey et al. 2015 | Retrospective MC analysis | N = 2247,15 centers | Ranked SI [%] pre-CI to post-CI | Unilat (quiet): 44% (± 29.0) to 58.5% Bilat (quiet): 42% (± 9.1) to 63% |
| (e6) | Häussler et al. 2019 | Retrospective SC cohort study | N = 20 | Points in TQ pre-CI to post-CI | 25.2 ± 18.7 to 17.3 ± 18.1 (p < 0.05) |
| N = 20 | SNR OLSA in noise | Without CI 0.43 dB. with CI: −1.8 dB (p > 0.005) | |||
| (34) | Helbig et al. 2008 | Retrospective SC cohort study | N = 9 | MSI in noise with SNR 5 | CI alone: 26%; EAS 53% (p = 0.02) |
| (35) | Lorens et al. 2008 | Retrospective SC cohort study | N = 11 | MSI in noise with SNR 0 [%] | CI alone: 18.6%; EAS 33.90% dB SNR: (p = 0.003) |
| (31) | Blamey et al. 2013 | Retrospective MC analysis | N = 2251,15 centers | Gain/time of ranked SI [%] | Shortest deafness group + 17.8% Longest deafness group + 9.4% |
| (38) | Gaylor et al. 2013 | Meta-analysis | N = na. 13 h | Change in QoL pre- to post-CI [%] ± SMD (95% CI) |
General: I2 = 39.8%; ± 1.05 (0.91–1.19) P = 0.01 Hearing-related: I2 = 57.5%; ± 1.2 (1.00–1.48) P = 0.05 |
| (37) | Amin et al. 2020 | Case control study | N1 = 47. 70–79 years N2 = 17: ≥ 80 years | Sentence test in quiet at 70 dB | No significant difference (P = 0.28) |
| (36) | Rohloff et al. 2017 | Case control study | N1 = 109: 18–69 years N2 = 53: ≥ 70 years |
Postoperative MSI in quiet | N1: 48.7%; N2: 51.8% p = 0.50. 95% CI: [−12.22; −5.98] |
| (5) | Völter et al. 2018 | Prospective SC study | N = 60 | M3 test (attention) pre-CI to 6 months post-CI |
Inverse efficiency performance: 935.73 (± 361.16) to 783.94 (± 350.67) P = 0.00027 |
| (e8) | Illg et al. 2017 | Retrospective SC study | N = 174 | Duration of CI use ISCED score CI vs. NH |
86.8% CI use > 11 h/day 2% CI nonusers ISCED (CI) 2.24 (± 0.59; range: 1–3) (t(1457) = 3723; p < 0.001) |
Bilat. bilateral CI treatment; CI. cochlear implant; MSI. monosyllabic speech intelligibility; ISCED. International Standard Classifcation of Education;
CI. confidence interval; MC. multicenter; NH. normal hearing; OLSA. Oldenburg sentence test; QoL. quality of life; SC. single center; SMD. standardized mean difference;
SNR. signal-to-noise ratio; SI. speech intelligibility; Unilat. unilateral CI treatment
Successful auditory rehabilitation can also be achieved in unilateral deafness (normal hearing on the contralateral side), meaning that CI treatment in the deaf ear:
Enables significantly better sound localization (improvement in angular error of 22.7 °)
Significantly improves speech intelligibility in noise (improvement in the signal-to-noise ratio [SNR] in the Oldenburg sentence test [OLSA] of 2.23 dB)
Significantly reduces impaired hearing due to accompanying tinnitus (26.3% improvement).
This can be evaluated using the internationally standardized Tinnitus Questionnaire (TQ) (20, e6).
Goal of auditory rehabilitation.
Today, the goal of auditory rehabilitation with CI is speech comprehension without the aid of lipreading, when telephoning, and in a noisy environment (background noise).
Particularly in patients with relevant residual hearing in the low frequency range, the combination of hearing aid and CI in one system (EAS) can significantly improve speech intelligibility by 15.6–25% in noise (33– 35). The effects of CI treatment are not limited only to younger patients, given that there is no significant difference in speech intelligibility with CI in the over-70s group compared to younger patients (18–69 years) (36, 37). In addition to an improvement in hearing and speech intelligibility, CI treatment is associated with a significant improvement in quality of life, meaning that cochlear implantation significantly improves both general (standardized mean difference [SMD]: 1.05; 95% confidence interval: (0.91; 1.19)]) and hearing-related quality of life (SMD: 1.24 [1.00; 1.48]) (38). Cognitive performance is also significantly improved following CI treatment in various subdomains such as “attention” (5). Thus the effects of cochlear implantation far exceed a mere improvement in hearing, since they have a positive impact on every aspect of the affected person’s social, emotional, and cognitive abilities.
In addition to the cost of implantation (diagnosis-related group D66Z: approximately €28 000), the costs of CI treatment for an adult also include other treatment components such as rehabilitation or aftercare, resulting in total costs of approximately €50,000. The lifetime costs of treatment in childhood can exceed more than three times that amount (39, 40).
Nevertheless, cost–benefit analyses show significant advantages for both children (40) and adults (e7) compared to hearing aids.
Auditory rehabilitation results with cochlear implants in children
The results of hearing and speech development in CI-treated children can only be assessed over the long term. In all, 86% of children with CI achieve open speech intelligibility for everyday sentences (median CAP, 5.5) and 98% still use the CI 17 years after implantation (e8). A total of 36% of children attended normal, integrated schools. These children also benefit from CI in long-term observations. For example, alone with their hearing, they are able to identify emotions (58%), distinguish emotions (89%), or identify the gender of speakers on the basis of voice (89%) (e8). Nevertheless, a survey using the International Standard Classification of Education levels (ISCED-97) shows that the average school-leaving qualifications of children with CI are significantly lower compared to the “normal population” (surveyed in the German General Social Survey [Allgemeine Bevölkerungsumfrage der Sozialwissenschaften], ALLBUS 2012). The average ISCED-97 level of the 174 CI-implant recipients surveyed was 2.24, while that of the the ALLBUS-97 2012 respondents was on average 3.723 (t [1457] = 3.723; p < 0.001) (e8). Given that CI treatment is now carried out as early on as in the first year of life, a further improvement in results can be expected in the future (29, e9, e10).
Complications
Effects of CI treatment.
The effects of cochlear implantation far exceed a mere improvement in hearing, since they have a positive impact on every aspect of the affected person’s social, emotional, and cognitive abilities.
Complications are overall rare, even when taking short-term and long-term events into account. Depending on the definition, rates of between 5.7% and 12.8% are reported (e4, e11), with the most common being implant defects (1.9–3.4%), dizziness (2.2–3.9%), and wound infections (1.9%). Severe perioperative complications in the form of facial paralysis are put at 0.1–0.6% of cases, and at below 0.1% for meningitis (e4, e11) (table 2).
Quality assurance in CI treatment
Since the center (otorhinolaryngology department) that performs cochlear implantation is considered the provider of the medical device (CI), it is primarily responsible for the structuring and organization of auditory rehabilitation (7).
The basic principles of quality assurance of CI treatment are described in the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V., AWMF) guideline (register no. 017–071) (8), which is currently being revised. Furthermore, in 2018, the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (Deutsche Gesellschaft für Hals-Nasen-Ohrenheilkunde, Kopf- und Halschirurgie) compiled the “White paper for Cochlea Implants (GERMANY)” (Weißbuch Cochlea-Implantat-Versorgung Deutschland) (7). In addition to uniform quality standards on process description, this document puts together, for the first time, structural recommendations (equipment, qualification, etc.) as well as a concept for setting up a nationwide CI register.
Conclusions for clinical practice
CI treatment results in significant improvements in speech intelligibility, cognitive performance, and quality of life in profoundly hearing-impaired or deaf children and adults. In centers specialized in CI treatment, the procedure has a low complication rate and a high benefit–risk ratio from childhood to old age. Future improvements in CI treatment as a result of optimized signal resolution are expected to arise from research activities, for example in the field of optogenetics and the use of growth factors.
Complications.
Depending on the definition, rates of between 5.7% and 12.8% are reported, with the most common being implant defects (1.9–3.4%), dizziness (2.2–3.9%), and wound infections (1.9%).
Further information on centers offering CI treatment in Germany can be obtained from the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (www.hno.org).
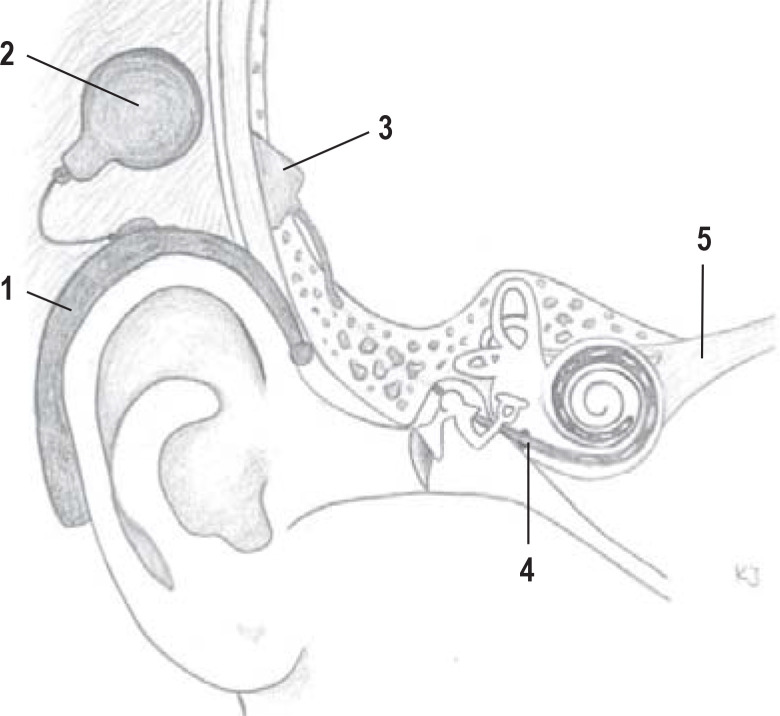
Figure.
Principle of a cochlear implant
The sound waves are picked up by a microphone in the audio processor (1), converted into electrical signals, and transmitted via the transmitter coil (2) to the receiver implant located beneath the skin (3). The electrical signals then stimulate—in a frequency-specific manner via the electrodes arranged on the electrode carrier (4)—the auditory nerve fibers (5).
Further information on CME.
Participation in the CME certification program is possible only via the Internet: cme.aerzteblatt.de. The submission deadline is 08.10.2021. Submissions by letter, email, or fax cannot be considered.
The completion time for all newly started CME units is 12 months. The results can be accessed 4 weeks following the start of the CME unit. Please note the respective submission deadline at: cme.aerzteblatt.de
This article has been certified by the North Rhine Academy for Continuing Medical Education. CME points can be managed using the uniform CME number (einheitliche Fortbildungsnummer, EFN). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or entered in “Meine Daten,” and consent must be given for results to be communicated. The 15-digit EFN can be found on the CME card (8027XXXXXXXXXXX).
Participation is possible at cme.aerzteblatt.de. The submission deadline is 8 October 2021. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the estimated prevalence of profound hearing loss or deafness in childhood and adolescence?
0.01%
0.1%
0.5%
1%
5%
Question 2
Which constellation represents a typical indication for cochlear implantation?
Congenital unilateral inner ear hearing loss bordering on deafness in a 45-year-old man
Moderate congenital sensorineural hearing loss in an 8-year-old girl
Slowly progressive bilateral sensorineural hearing loss meanwhile bordering on deafness in a 70-year-old woman experiencing no improvement with conventional hearing aids
Bilateral profound hearing loss combined with low-grade conduction components in a 62-year-old man
Congenital bilateral sensorineural hearing loss bordering on deafness in a 55-year-old woman
Question 3
What is meant by a modern cochlear implant?
An electronic inner ear prosthetic device that electrically stimulates the auditory nerve
A hearing amplifier with stethoscope headphones and ring loop function
An inner ear prosthetic device with hearing amplifier
An ultrasound acoustic amplifier
A device worn behind the ear with adjustable noise suppression
Question 4
How does one objectively establish the indication for cochlear implantation in infants?
By means of head X-ray
By means of magnetic resonance imaging of the head
Using the speech intelligibility test
Using a conventional hearing test
Using BERA (brainstem evoked response audiometry)
Question 5
Which aspects are of particular relevance in the pre-diagnostic work-up for cochlear implantation in childhood?
Intelligence test and X-ray of the skull in two planes
Patient history regarding hearing and speech development, as well as objective hearing tests
Hearing history and cranial computed tomography
Patient social history
Age-appropriate speech intelligibility test and careful inspection of the outer ear
Question 6
At what age is CI treatment recommended in infants born with bilateral hearing loss?
Within the first year of life
Once language acquisition has completely failed (at around the age of 4 years)
At primary school age
When the child is able to give consent
Once the child has reached the age of majority
Question 7
Up to what age can auditory rehabilitation with a cochlear implant be beneficial?
Since there are no age limits, elderly patients (>85 years) can also be successfully treated.
Patients aged over 65 years should no longer be offered cochlear implant surgery.
Auditory rehabilitation with a cochlear implant can only be carried out during middle age (40–65 years).
Cochlear implant treatment is beneficial only up to the age of around 20 yearsl.
It is only beneficial in children with congenital deafness.
Question 8
When should treatment with a cochlear implant ideally be carried out following the diagnosis of hearing loss due to meningitis/labyrinthitis/trauma?
Immediately
As soon as possible within approximately 4–6 weeks
Upon confirmation of deafness after around 6 months
Upon confirmation of deafness after around 12 months
Cochlear implantation is contraindicated following meningitis.
Question 9
How should unilateral profound hearing loss bordering on deafness be treated if there is normal hearing in the contralateral ear?
With a bilateral cochlear implant
With electric acoustic stimulation
With a unilateral cochlear implant
With a middle ear implant
With bimodal treatment (cochlea implant + hearing aid in the contralateral ear)
Question 10
When establishing the indication for cochlear implant treatment, which audiometric parameter is usually given special consideration in open-field measurements using the Freiburg speech audiogram in adults with postlingually acquired hearing loss?
Speech intelligibility in noise at 75 dB
Syllable intelligibility in quiet at 50 dB
Recognition of numerals at 60 dB
Monosyllable intelligibility in quiet at 65 dB
Speech intelligibility at 55 dB with 40-dB background noise
► Participation is only possible via: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Christine Rye.
Acknowledgments
We would like to thank Katharina Johannsen at the Bochum University ENT Department for producing the figures.
Footnotes
Conflict of interest statement
Prof. Dazert received reimbursement of congress participation fees and accommodation costs, honoraria for preparing continuing medical education events, as well as funds for a research project of his own initiation and support for the organization of surgery courses from Advanced Bionics, Cochlear, and Med-EL.
PD Thomas received reimbursement of congress participation fees and accommodation costs, honoraria for preparing continuing medical education events, as well as support for the organization of surgery courses from Advanced Bionics, Cochlear, and Med-EL.
Dr. Loth received funds for a research project of his own initiation from Med-EL.
Prof. Zahnert received funds for a research project of his own initiation from Med-EL and Cochlear.
Prof. Stöver received consultancy fees from MED-EL and Advance Bionics. He was reimbursed for congress participation fees by MED-EL and Cochlear. MED-EL, Advance Bionics, and Cochlear reimbursed his accommodation costs. He received honoraria for preparing continuing medical education events from MED-EL. Med-EL, Cochlear, and Advanced Bionics made funds available to him for a research project of his own initiation. He received funds to conduct clinical trials from Cochlear and MED-EL.
References
- 1.Löhler J, Walther LE, Hansen F, et al. The prevalence of hearing loss and use of hearing aids among adults in Germany: a systematic review. Eur Arch Otolaryngol. 2019;276:945–956. doi: 10.1007/s00405-019-05312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmucker C, Kapp P, Motschall E, Loehler J, Meerpohl JJ. Prevalence of hearing loss and use of hearing aids among children and adolescents in Germany: a systematic review. BMC Public Health. 2019;19 doi: 10.1186/s12889-019-7602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Löhler J, Cebulla M, Shehata-Dieler W, Volkenstein S, Völter C, Walther LE. Hearing impairment in old age-detection, treatment, and associated risks. Dtsch Arztebl Int. 2019;116:301–310. doi: 10.3238/arztebl.2019.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin FR, Albert M. Hearing loss and dementia-who is listening? Aging Ment Health. 2014;18:671–673. doi: 10.1080/13607863.2014.915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Völter C, Götze L, Dazert S, Falkenstein M, Thomas JP. Can cochlear implantation improve neurocognition in the aging population? Clin Interv Aging. 2018;13:701–712. doi: 10.2147/CIA.S160517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löhler J, Lehmann M, Segler V, et al. The Mini-Audio-Test (MAT) - a screening method on hearing impairment to be used by general practitioners and specialized physicians. Laryngorhinootologie. 2019;98:27–34. doi: 10.1055/a-0805-5741. [DOI] [PubMed] [Google Scholar]
- 7.DGHNO (Deutsche Gesellschaft für Hals-Nasen-Ohrenheilkunde, Kopf- und Hals-Chirurgie) Weißbuch Cochlea-Implantat(CI)-Versorgung. https://cdn.hno.org/media/PDF/ci-weissbuch-und-register-dghno-1-auflage-stand-04-2018.pdf (last accessed on 22 February 2020) [Google Scholar]
- 8.AWMF. Leitlinie Cochlea-Implantat Versorgung der DGHNO 2020 (in press) www.awmf.org/leitlinien/detail/ll/017-071.html (last accessed on 19 July 2020) [Google Scholar]
- 9.Hoff S, Ryan M, Thomas D, et al. Safety and effectiveness of cochlear implantation of young children, including those with complicating conditions. Otol Neurotol. 2019;40:454–463. doi: 10.1097/MAO.0000000000002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leigh J, Dettman S, Dowell R, Sarant J. Evidence-based approach for making cochlear implant recommendations for infants with residual hearing. Ear Hear. 2011;32:313–322. doi: 10.1097/AUD.0b013e3182008b1c. [DOI] [PubMed] [Google Scholar]
- 11.Vickers D, Summerfield Q, Lovett R. Candidacy criteria for paediatric bilateral cochlear implantation in the United Kingdom. Cochlear Implants Int. 2015;16:48–49. doi: 10.1179/1467010014Z.000000000235. [DOI] [PubMed] [Google Scholar]
- 12.Kral K, Lang-Roth R, Hilger N, Streicher B. Do early cochlear-implanted toddlers show a better speech development than later implanted children? Laryngorhinootologie. 2017;96:160–167. doi: 10.1055/s-0042-109613. [DOI] [PubMed] [Google Scholar]
- 13.Chiossi JSC, Hyppolito MA. Effects of residual hearing on cochlear implant outcomes in children: A systematic review. Int J Pediatr Otorhinolaryngol. 2017;100:119–127. doi: 10.1016/j.ijporl.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Hoppe U, Hocke T, Hast A, Iro H. Maximum monosyllabic score as a predictor for cochlear implant outcome. HNO. 2019;67:199–206. doi: 10.1007/s00106-018-0605-3. [DOI] [PubMed] [Google Scholar]
- 15.Dowel RC. The case for earlier cochlear implantation in postlingually deaf adults. Int J Audiol. 2016;55:51–56. doi: 10.3109/14992027.2015.1128125. [DOI] [PubMed] [Google Scholar]
- 16.Lammers MJW, Versnel H, Topsakal V, van Zanten GA, Grolman W. Predicting performance and non-use in prelingually deaf and late-implanted cochlear implant users. Otol Neurotol. 2018;39:e436–e442. doi: 10.1097/MAO.0000000000001828. [DOI] [PubMed] [Google Scholar]
- 17.Rousset A, Dowell R, Leigh J. Receptive language as a predictor of cochlear implant outcome for prelingually deaf adults. Int J Audiol. 2016;55:24–30. doi: 10.3109/14992027.2016.1157269. [DOI] [PubMed] [Google Scholar]
- 18.Sparreboom M, Langereis MC, Snik AF, Mylanus EA. Long-term outcomes on spatial hearing, speech recognition and receptive vocabulary after sequential bilateral cochlear implantion in children. Res Dev Disabil. 2015;36C:328–337. doi: 10.1016/j.ridd.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Van de Heyning P, Távora-Vieira D, Mertens G, et al. Towards a unified testing framework for single-sided deafness studies: a consensus paper. Audiol Neurootol. 2017;21:391–398. doi: 10.1159/000455058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arndt S, Laszig R, Aschendorff A, Hassepass F, Beck R, Wesarg T. Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. HNO. 2017;65(2):98–108. doi: 10.1007/s00106-016-0297-5. [DOI] [PubMed] [Google Scholar]
- 21.Sladen DP, Carlsin ML, Dowling BP, et al. Early outcomes after cochlear implantation for adults and children with unilateral hearing loss. Laryngoscope. 20171;27:1683–1688. doi: 10.1002/lary.26337. [DOI] [PubMed] [Google Scholar]
- 22.Laszig R, Aschendorff A, Beck R, et al. Long-term functional outcomes of cochlear implants in children. HNO. 2009;57:657–662. doi: 10.1007/s00106-009-1939-7. [DOI] [PubMed] [Google Scholar]
- 23.Briggs RJ, Tykocinski M, Xu J, et al. Comparison of round window and cochleostomy approaches with a prototype hearing preservation electrode. Audiol Neurootol. 2006;11:42–48. doi: 10.1159/000095613. [DOI] [PubMed] [Google Scholar]
- 24.Sato M, Baumhoff P, Tillein J, Kral A. Physiological mechanisms in combined, electric-acoustic stimulation. Otol Neurotol. 2017;38:e215–e223. doi: 10.1097/MAO.0000000000001428. [DOI] [PubMed] [Google Scholar]
- 25.Lenarz T, James C, Cuda D, et al. European multi-centre study of the Nucleus Hybrid L24 cochlear implant. Int J Audiol. 2013;52:838–848. doi: 10.3109/14992027.2013.802032. [DOI] [PubMed] [Google Scholar]
- 26.Hoppe U, Liebscher T, Hornung J. Anpassen von Cochlea-Implantatsystemen. HNO. 2017;65:546–551. doi: 10.1007/s00106-016-0226-7. [DOI] [PubMed] [Google Scholar]
- 27.Zeh R, Baumann U. Stationäre Rehabilitationsmaßnahmen bei erwachsenen CI-Trägern: Ergebnisse in Abhängigkeit von der Dauer der Taubheit, Nutzungsdauer und Alter. HNO. 2015;63:557–576. doi: 10.1007/s00106-015-0037-2. [DOI] [PubMed] [Google Scholar]
- 28.Diller G. (Re)habilitation nach Versorgung mit einem Kochleaimplantat. HNO. 2009;57:649–656. doi: 10.1007/s00106-009-1922-3. [DOI] [PubMed] [Google Scholar]
- 29.López-Higes R, Gallego C, Martín-Aragoneses MT, Melle N. Morpho-syntactic reading comprehension in children with early and late cochlear implants. J Deaf Stud Deaf Educ. 2015;20:136–146. doi: 10.1093/deafed/env004. [DOI] [PubMed] [Google Scholar]
- 30.Boisvert I, Reis M, Au A, Cowan R, Dowell RC. Cochlear implantation outcomes in adults: A scoping review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232421. e0232421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blamey P, Artieres F, Baskent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol. 2013;18:36–47. doi: 10.1159/000343189. [DOI] [PubMed] [Google Scholar]
- 32.Blamey PJ, Maat B, Başkent D, et al. A retrospective multicenter study comparing speech perception outcomes for bilateral implantation and bimodal rehabilitation. Ear Hear. 2015;36:408–416. doi: 10.1097/AUD.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 33.von Ilberg CA, Baumann U, Kiefer J, Tillein J, Adunka OF. Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiol Neurootol. 2011;16:1–30. doi: 10.1159/000327765. [DOI] [PubMed] [Google Scholar]
- 34.Helbig S, Baumann U, Helbig M, von Malsen-Waldkirch N, Gstoettner W. A new combined speech processor for electric and acoustic stimulation-eight months experience. ORL J Otorhinolaryngol Relat Spec. 2008;70:359–365. doi: 10.1159/000163031. [DOI] [PubMed] [Google Scholar]
- 35.Lorens A, Polak M, Piotrowska A, Skarzynski H. Outcomes of treatment of partial deafness with cochlear implantation: a DUET study. Laryngoscope. 2008;118:288–294. doi: 10.1097/MLG.0b013e3181598887. [DOI] [PubMed] [Google Scholar]
- 36.Rohloff K, Koopmann M, Wei D, Rudack C, Savvas E. Cochlear implantation in the elderly: Does age matter? Otol Neurotol. 2017;38:54–59. doi: 10.1097/MAO.0000000000001262. [DOI] [PubMed] [Google Scholar]
- 37.Amin N, Wong G, Nunn T, Jiang D, Pai I. The outcomes of cochlear implantation in elderly patients: a single united kingdom center experience. Ear Nose Throat J. 2020 doi: 10.1177/0145561320910662. 145561320910662 online ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Gaylor JM, Raman G, Chung M, et al. Cochlear implantation in adults: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139:265–272. doi: 10.1001/jamaoto.2013.1744. [DOI] [PubMed] [Google Scholar]
- 39.Raths S, Lenarz T, Lesinski-Schiedat A, Flessa S. Kostenanalyse der unilateralen Cochlea-Implantatversorgung bei Erwachsenen. Laryngorhinootologie. 2016;95:251–257. doi: 10.1055/s-0035-1555936. [DOI] [PubMed] [Google Scholar]
- 40.Schulze-Gattermann H, Illg A, Lesinski-Schiedat A, Schoenermark M, Bertram B, Lenarz T. Kosten-Nutzen-Analyse der Cochlea-Implantation bei Kindern. Laryngorhinootologie. 2003;82:322–329. doi: 10.1055/s-2003-39725. [DOI] [PubMed] [Google Scholar]
- E1.Leitlinie Periphere Hörstörungen im Kindesalter 2013, Langfassung, in Überarbeitung. www.awmf.org/leitlinien/detail/ll/049-010.html (last accessed on 23 February 2020) [Google Scholar]
- E2.Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 20121;72:369–371. doi: 10.1001/archinternmed.2011.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Müller A, Hocke T, Mir-Salim P. Intraoperative findings on ECAP-measurement: normal or special case? Int J Audiol. 2015;54:257–264. doi: 10.3109/14992027.2014.969410. [DOI] [PubMed] [Google Scholar]
- E4.Carlson ML. Cochlear implantation in adults. N Engl J Med. 2020;382:1531–1542. doi: 10.1056/NEJMra1904407. [DOI] [PubMed] [Google Scholar]
- E5.Hoppe U, Hocke T, Hast A, Iro H. Maximum preimplantation monosyllabic score as predictor of cochlear implant outcome. HNO. 2019;67:62–68. doi: 10.1007/s00106-019-0648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Häußler SM, Knopke S, Dudka S, Gräbel S, Ketterer MC, Battmer RD, Ernst A, Olze H. Improvement in tinnitus distress, health-related quality of life and psychological comorbidities by cochlear implantation in single-sided deaf patients. HNO. 2019;67:863–873. doi: 10.1007/s00106-019-0706-7. [DOI] [PubMed] [Google Scholar]
- E7.Laske RD, Dreyfuss M, Stulman A, Veraguth D, Huber AM, Röösli C. Age dependent cost-effectiveness of cochlear implantation in adults. Is there an age related cut-off? Otol Neurotol. 2019;40:892–899. doi: 10.1097/MAO.0000000000002275. [DOI] [PubMed] [Google Scholar]
- E8.Illg A, Haack M, Lesinski-Schiedat A, Büchner A, Lenarz T. Long-term outcomes, education, and occupational level in cochlear implant recipients who were implanted in childhood. Ear Hear. 2017;38:577–587. doi: 10.1097/AUD.0000000000000423. [DOI] [PubMed] [Google Scholar]
- E9.Geers AE, Davidson LS, Uchanski RM, Nicholas JG. Interdependence of linguistic and indexical speech perception skills in school-age children with early cochlear implantation. Ear Hear. 2013;34:562–574. doi: 10.1097/AUD.0b013e31828d2bd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Karltorp E, Eklöf M, Östlund E, Asp F, Tideholm B, Löfkvist U. Cochlear implants before 9 months of age led to more natural spoken language development without increased surgical risks. Acta Paediatr. 2020;109:332–341. doi: 10.1111/apa.14954. [DOI] [PubMed] [Google Scholar]
- E11.Terry B, Kelt RE, Jeyakumar A. Delayed complications after cochlear implantation. JAMA Otolaryngol Head Neck Surg. 2015;141:1012–1017. doi: 10.1001/jamaoto.2015.2154. [DOI] [PubMed] [Google Scholar]