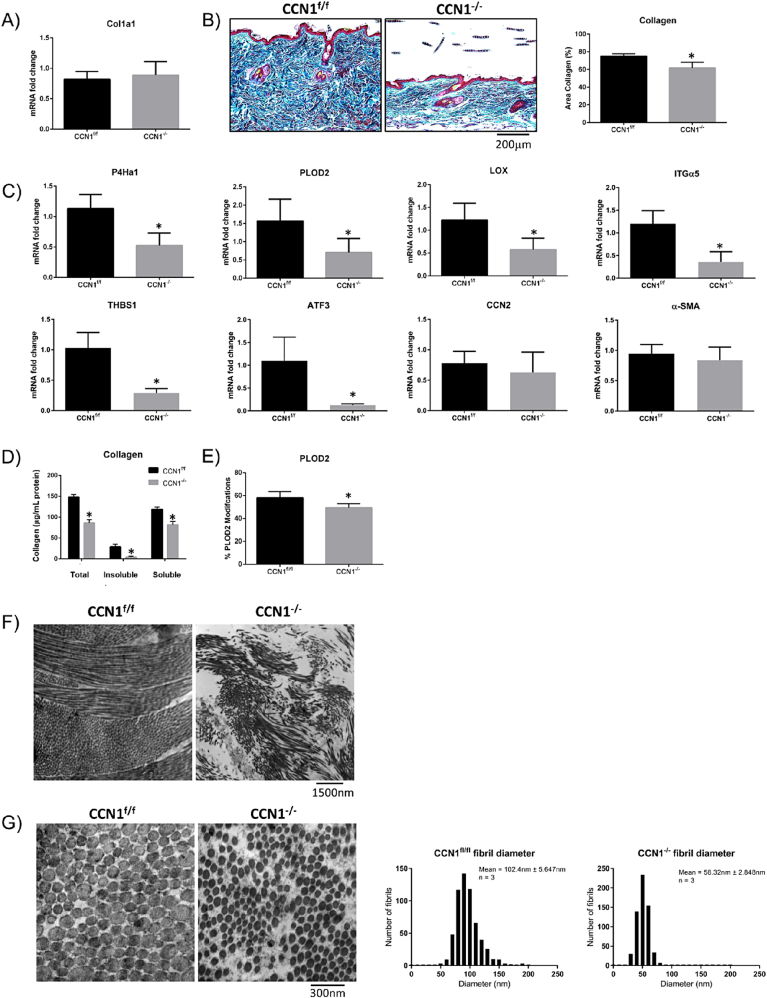
Fig. 2.
Loss of CCN1 from fibroblasts resulted in decreased in collagen stability, but no difference in collagen mRNA expression. A) RNA from skin tissue showed no changes in collagen type I mRNA expression. (Graph represents mean fold change +/− SD; Student's t-test; n = 4). B) Skin tissue showed a significant decrease in collagen (p = 0.0014) shown by trichrome staining. (Representative images shown; Graph shows mean area of collagen present in images +/− SD; Student's t-test; n = 4). C) Skin tissue from CCN1-deficient skin showed a significant decrease in expression of mRNAs encoding cross-linking enzymes P4Ha1 (P = 0.0009), PLOD2 (P = 0.0115) and LOX (P = 0.0046), and mRNAs encoding the ECM-promoting proteins integrin alpha5 (ITGα5, p = 0.0002), thrombospondin-1 (THBS1, p < 0.0001) and ATF3 (p = 0.0325). There were no changes observed in the fibrotic markers CCN2 and α-smooth muscle actin (α-SMA) in CCN1-deficient skin tissue. (Graphs represent mean mRNA fold change +/− SD; Student's t-test; n = 4). Note: total skin tissue was examined. D) Significantly less total (p > 0.0001), insoluble (p = 0.0003) and soluble (p = 0.0003) collagen were present in the skin of mice lacking CCN1 as shown by a hydroxyproline assay. (Graph represents mean collagen content (ug/mg) +/− SD; Student's t-tests; n = 4). E) Proteomics using mass spectrometry revealed a significant decrease in PLOD2 (p = 0.0364)-generated collagen crosslinks in tissue of CCN1−/− mice. (Graph represents mean modifications +/− SD; Student's t-test; n = 4). F.G) electron microscopy of sections of CCN1f/f and CCN1−/− skin. Images show a significant decrease (p = 0.0022) in collagen fibril diameter in the CCN1−/− mouse dermis. Inset shows mean fibril diameter +/− SD; Student's t-test (n = 3).