Abstract
Introduction
While unfavourable changes in High Density Lipoprotein (HDL)-cholesterol appear to be a consequence of COVID-19, the reverse has been little-studied. Our objective was to test whether HDL-cholesterol within the normal range is associated with subsequent COVID-19 hospitalisation.
Design
We examined 317,306 participants in the prospective UK Biobank study with complete data on HDL-cholesterol and covariates at baseline (2006–2010). Follow-up for COVID-19 status was via hospitalisation records in England (16th March and 31st May 2020). Death certificates for the period 1st March to 30th September 2020 with an underlying cause denoted as COVID-19 (emergency ICD-10 code U07.1) were also utilised.
Results
Lower COVID-19 hospitalisation risk was apparent in people with higher level of HDL-cholesterol, adjusting for factors including health behaviours, inflammatory markers, and socio-economic status. The association appeared to be linear so that for each 0.2 mmol/L increase in HDL-cholesterol, the odds ratio for COVID-19 hospitalisation was 0.91 (95% confidence interval: 0.86, 0.96). A similar pattern of association was apparent when deaths from COVID-19 was the outcome of interest.
Conclusions
Adequately high levels of HDL-cholesterol are associated with a lower risk of severe COVID-19.
Introduction
Fat metabolism may be important in response to viral infection and invasion such that lipids act as viral receptors in addition to fusion and entry cofactors for viruses in host cells (1). In particular, high density lipoprotein (HDL)-cholesterol concentrations appear to drop in the presence of coronavirus disease 2019 (COVID-19), whereby, relative to healthy controls, patients with COVID-19 – the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – have lower levels of HDL-cholesterol concentrations in conjunction with acute elevation in systemic inflammatory markers (2–4). It has also been widely documented that pre-existing cardiovascular disease and risk factors are associated with worse prognosis in COVID-19 patients (5).
While unfavourable changes in HDL-cholesterol appear to be a consequence of infection with SARS-Cov-2, the reverse has been little examined. In a sample of 228 hospitalized COVID-19 patients in China, those with low HDL-cholesterol within 24 hours of hospital admission were more likely to present a severe form of the disease (4). However, in that study lipid levels before onset of COVID-19 were unknown. In cohorts of apparently healthy individuals, lower baseline levels of HDL-cholesterol are associated with greater risk of subsequent infectious disease several years later (6), and there is a suggestion from Mendelian randomisation studies that this effect is likely to be causal (7). Potential mechanisms include HDL-mediated sequestration of pathogen-associated lipids, and regulation of the proliferation, maturation and function of immune cells, resulting in neutralization/clearance of pro-inflammatory endotoxins (8). We have shown that an unfavourable risk factor profile assessed pre-pandemic – low HDL included – is associated with a higher risk of hospitalization for COVID-19 up to 14 years later (9). Whether HDL-cholesterol within the normal range have predictive capacity for COVID-19 hospitalisations has yet to be examined and this is the purpose of the present study. With deaths from the disease also now available in the present dataset, we augment our analyses with these new data.
Methods
We used data from the UK Biobank prospective cohort study (10). Baseline data collection took place between 2006 and 2010 across centres in the UK, yielding a sample of 502,655 people (448,919 from England) aged 40–69 years. Ethical approval was provided by the North-West Multi-centre Research Ethics Committee (11/NW/0382; 16/NW/0274).
At baseline, non-fasting venous blood samples were drawn and assayed for total cholesterol, HDL, and triglycerides using a Beckman Coulter AU5800 analytical platform. Low density lipoprotein (LDL)-cholesterol values were calculated using the Friedewald equation (11). Total blood count (leukocyte, platelet, haemoglobin) as markers of immune function were analysed using an automated Coulter LH 750. Physician-diagnosed cardiovascular disease (heart attack, angina, stroke), diabetes, cholesterol-lowering drugs use, cigarette smoking, alcohol intake, highest educational attainment, ethnicity, number of people living in the household, and physical activity in the prior month were self-reported. Body mass index was based on height and weight measurements. Hypertension was defined as elevated measured blood pressure (≥140/90 mmHg) or use of anti-hypertensive medication. Townsend index of neighbourhood deprivation was based on postcode linkage. Provided by Public Health England, data on COVID-19 status in hospitalised patients in England covered the period 16th March until 31st May 2020. Tests were performed in accredited laboratories on samples from combined nose/throat swabs using real time polymerase chain reaction. Death certificates for the period 1st March to 30th September 2020 with an underlying cause denoted as COVID-19 (emergency ICD-10 code U07.1) were also utilised.
We used logistic regression analyses to summarise the relation between HDL-cholesterol and later COVID-19 hospitalisation or death. In these analyses, we used narrow (0.2 mmol/L) increments to examine the ‘shape’ of this relationship. Models included the following covariates: age (continuous) , sex, ethnicity (Asian, Black, White, Other), education (Higher education yes/no), number in household (living alone, 2 people, 3 people, 4 people or more), Townsend deprivation index (quintiles), body mass index (continuous), leisure time physical activity (inactive, active below guideline, meets guideline), alcohol intake (abstainer, moderate drinker, heavy drinker), smoking habit (never, ex-, current smoker), diagnosed diabetes, cardiovascular disease, hypertension, cholesterol-lowering drug use (yes/no), LDL-cholesterol, triglycerides, haemoglobin, white blood cell, and platelet count (all five biomarkers modelled as continuous). In a first model we used HDL-cholesterol as a continuous variable, and in a second model we created a categorical variable with 8 categories (<1.0 (reference category), 1.0 to <1.2, 1.2 to <1.4, 1.4 to <1.6, 1.6 to <1.8, 1.8 to <2.0, 2.0 to <2.2, 2.2 mmol/L).
Results
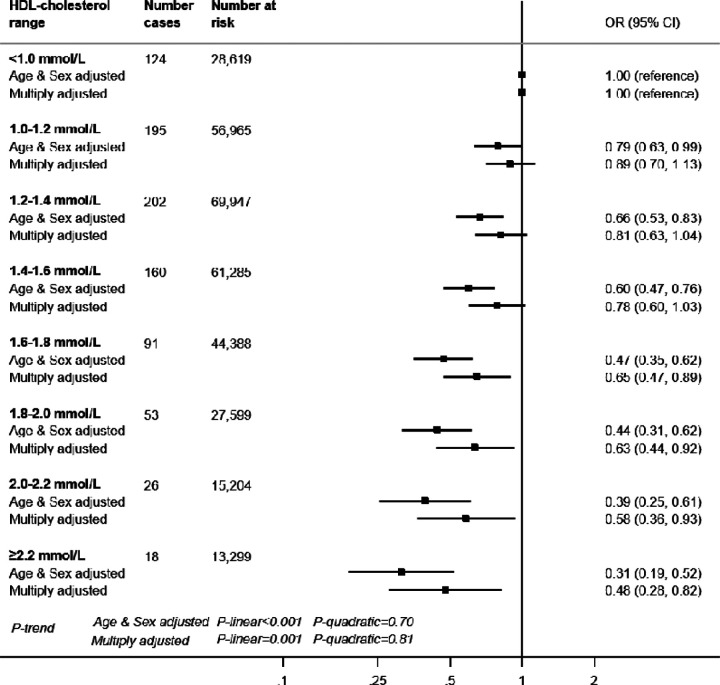
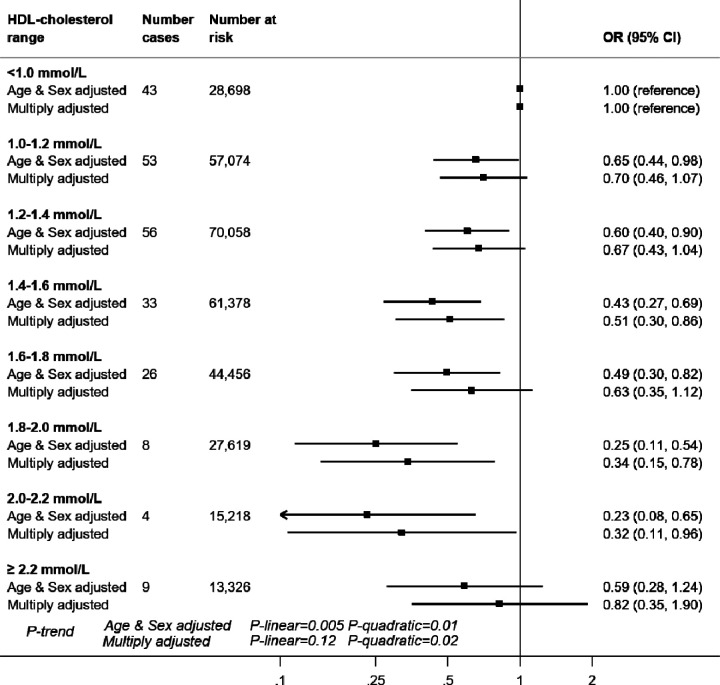
In 317,306 (171,466 women) participants with complete data on baseline covariates, there were 869 hospitalisations for COVID-19 after a median follow-up of 14 years (all cases were between 16th March and 31st May 2020). In age- and sex-adjusted analyses, there was a stepwise relationship between HDL-cholesterol and COVID-19 hospitalisation (p-value linear trend <0.001) such that lower infection risk was apparent in people with higher level of this cholesterol fraction (Figure 1). For a 0.2 mmol/L HDL-cholesterol increase, the odds ratio (OR) was 0.85 (95% confidence interval: 0.82, 0.89). Adjusting for an array of confounding factors - health behaviours, inflammatory markers, and socio-economic status - resulted in a shallower gradient but the linear associations remained: OR0.2 mmol/L increase = 0.91 (95% confidence interval: 0.86, 0.96). In 317,827 participants who were alive on March 1st 2020, there were 232 cases of COVID-19 deaths. The relationship between HDL-cholesterol and mortality due to COVID-19 also showed a negative association with an OR for a 0.2 mmol/L HDL-cholesterol increase of 0.90 (95% CI: 0.81, 1.00). However, as seen on Figure 2, the reduced risk was not apparent at very high values (≥2.2 mmol/L) of HDL-cholesterol compared to values <1.0 mmol/L (adjusted OR =0.82 [95%CI: 0.35, 1.90]), p-value for quadratic trend = 0.02).
Figure 1. HDL-cholesterol and risk of subsequent hospitalisation for COVID-19, UK Biobank, n=317,306.
Multiply adjusted odds ratios were from logistic regression models including age (continuous) , sex, ethnicity (Asian, Black, White, Other), education (Higher education yes/no), number in household (living alone, 2 people, 3 people, 4 people or more), Townsend deprivation index (quintiles), body mass index (continuous), leisure time physical activity (inactive, active below guideline, meets guideline), alcohol intake (abstainer, moderate drinker, heavy drinker), smoking habit (never, ex-, current smoker), diagnosed diabetes, cardiovascular disease, hypertension, cholesterol-lowering drug use (yes/no), LDL-cholesterol, triglycerides, haemoglobin, white blood cell, and platelet count (all five biomarkers modelled as continuous).
Figure 2. HDL-cholesterol and risk of death by COVID-19, UK Biobank, n=317,827.
Multiply adjusted odds ratios were from logistic regression models including age (continuous) , sex, ethnicity (Asian, Black, White, Other), education (Higher education yes/no), number in household (living alone, 2 people, 3 people, 4 people or more), Townsend deprivation index (quintiles), body mass index (continuous), leisure time physical activity (inactive, active below guideline, meets guideline), alcohol intake (abstainer, moderate drinker, heavy drinker), smoking habit (never, ex-, current smoker), diagnosed diabetes, cardiovascular disease, hypertension, cholesterol-lowering drug use (yes/no), LDL-cholesterol, triglycerides, haemoglobin, white blood cell, and platelet count (all five biomarkers modelled as continuous).
Discussion
To the best of our knowledge, this is the first aetiological study to examine the relationship between usual HDL-cholesterol levels and subsequent risk of COVID-19 hospitalisation. Evidence from the UK Biobank showed the same inverse association between baseline HDL and hospitalisations for any infectious disease up to 10 years later (7), while investigators on another large-scale study using two Danish community-based cohorts with up to 20 years of follow-up reported a ‘U’-shaped relationship whereby the greatest risk was apparent at opposing ends of the HDL-cholesterol continuum (6).
Potential mechanisms for the HDL–COVID-19 hospitalisation gradient include anti-inflammatory properties of the lipoprotein which are related to lower risk of cardiovascular disease in humans (12), particularly relevant in COVID-19 complications. Further, HDL can act as a direct modulator of immunity by inhibiting haematopoeietic stem cells proliferation and affecting the activity and function of immune cells by changing the cholesterol content of the lipid rafts in immune cell membranes (8). A direct binding and neutralization of viral particles by HDL may also explain the observed association (13).
The strengths of this study include the measurement of biomarkers that preceded the onset of COVID-19, and linkage to hospital data in a well-characterised population. Results were for severe COVID-19 manifestations requiring hospitalisation, and supported by analyses in which death from this disease was the outcome of interest. That UK Biobank participants represent only 5.5% of the target population means that the present data cannot be used to estimate prevalence or incidence in the general population, although established risk factor associations appear generalisable (14). HDL-cholesterol levels were measured 14 years before COVID-19 case assessment, raising concerns about their utility for current values, however, in a reassessment 4.4 years later in a subsample of the present population (n=13,430) they showed high test-retest stability (correlation coefficient 0.85, p<0.001).
In conclusion, we report a linear association between higher levels of HDL-cholesterol and lower risk of severe COVID-19.
Funding:
CL is supported by the Beatriu de Pinós postdoctoral programme of the Government of Catalonia’s Secretariat for Universities and Research of the Ministry of Economy and Knowledge (2017-BP-00021); GDB is supported by the Medical Research Council (MR/P023444/1) and the US National Institute on Aging (1R56AG052519-01; 1R01AG052519-01A1); MH through a joint award from the Economic Social Research Council and Medical Research Council (RES-579-47-0001).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Abu-Farha M, et al. (2020) The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int J Mol Sci 21(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei X, et al. (2020) Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol 14(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorokin AV, et al. (2020) COVID-19-Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J 34(8):9843–9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G, et al. (2020) Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis 19(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Li R, Lu Z, & Huang Y (2020) Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 12(7):6049–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madsen CM, Varbo A, Tybjaerg-Hansen A, Frikke-Schmidt R, & Nordestgaard BG (2018) U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J 39(14):1181–1190. [DOI] [PubMed] [Google Scholar]
- 7.Trinder M, Walley KR, Boyd JH, & Brunham LR (2020) Causal Inference for Genetically Determined Levels of High-Density Lipoprotein Cholesterol and Risk of Infectious Disease. Arterioscler Thromb Vasc Biol 40(1):267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catapano AL, Pirillo A, Bonacina F, & Norata GD (2014) HDL in innate and adaptive immunity. Cardiovasc Res 103(3):372–383. [DOI] [PubMed] [Google Scholar]
- 9.Batty GD & Hamer M (2020) Vascular risk factors, Framingham risk score, and COVID-19: community-based cohort study. Cardiovasc Res 116(10):1664–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudlow C, et al. (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS medicine 12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, & Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502. [PubMed] [Google Scholar]
- 12.Soria-Florido MT, Schroder H, Grau M, Fito M, & Lassale C (2020) High density lipoprotein functionality and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 302:36–42. [DOI] [PubMed] [Google Scholar]
- 13.Kane JP, Hardman DA, Dimpfl JC, & Levy JA (1979) Apolipoprotein is responsible for neutralization of xenotropic type C virus by mouse serum. Proc Natl Acad Sci U S A 76(11):5957–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batty GD, Gale CR, Kivimaki M, Deary IJ, & Bell S (2020) Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ 368:m131. [DOI] [PMC free article] [PubMed] [Google Scholar]