Abstract
COVID-19 vaccines currently approved in the United States require two doses, administered three to four weeks apart. Constraints in vaccine supply and distribution capacity, together with the rise of COVID-19 cases and hospitalizations, have sparked a policy debate on whether to vaccinate more individuals with the first dose of available vaccines and delay the second dose, or to continue with the recommended two-dose series as tested in clinical trials. We developed an agent-based model of COVID-19 transmission to compare the impact of these two vaccination strategies, while varying the temporal waning of vaccine efficacy against disease following the first dose, vaccine efficacy against infection, and the level of pre-existing immunity in the population. Our results show that for Moderna vaccines with 80% efficacy following the first dose, a delay of 9–12 weeks could enhance the program effectiveness and prevent additional infections, hospitalizations, and deaths, compared to a 4-week interval between the doses. However, for Pfizer-BioNTech vaccines with demonstrated efficacy of 52% after the first dose, there was no clear advantage for delaying the second dose beyond the 3-week tested schedule, unless the efficacy of the first dose did not wane over time. Our findings underscore the importance of quantifying the durability of vaccine-induced protection after the first dose as well as vaccine efficacy against infection in order to determine the optimal time interval between the two doses.
Introduction
The rapid spread of coronavirus disease 2019 (COVID-19) continues to ravage global health and suppress economic activity despite the range of mitigation measures implemented by countries worldwide. A number of vaccines, including those developed by Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca, have received emergency use authorization from regulatory bodies in different countries [1]. Clinical trials have demonstrated that these vaccines can provide high levels of protection against symptomatic and severe disease with two doses administered three to four weeks apart [2,3]. In contrast to the remarkable speed of development, vaccine delivery has proven to be challenging due to supply shortages and limited distribution capacity [4,5].
Recent spikes in cases and hospitalizations in the United Kingdom (UK), the United States (US), and Canada have led to a public health conundrum regarding whether to vaccinate more individuals with the first dose of available vaccines and delay the second dose, or to prioritize completion of the two-dose series based on tested schedules in clinical trials [6–9]. Broader population-level protection against COVID-19 in a delayed second dose (DSD) strategy, even with lower individual-level efficacy from the first dose in the short term, may improve the impact of vaccination compared to the recommended two-dose strategy that provides more complete protection to a smaller subset of the population [6,9]. However, the conditions under which this improvement is achievable remain unexamined, such as the durability of first dose efficacy and protection against infection [10–12].
Here, we employed an agent-based model of COVID-19 transmission and vaccination to compare the epidemiological impact of tested and DSD vaccination schedules, considering a range of pre-existing immunity accrued since the emergence of COVID-19. We determined the optimal timing for administering the second dose based on the trade-offs between potential temporal decline in efficacy of the first dose against infection and disease, and the maximum protection level conferred by a DSD. For Moderna’s two-dose vaccine, we show that a DSD strategy would significantly outperform the recommended 28-day interval between doses in terms of reducing the number of infections, hospitalizations and deaths. However, such benefits with Pfizer-BioNTech vaccines depend on the level of pre-existing immunity and the efficacy of the first dose over time.
Methods
Model structure
We extended our previous model of agent-based COVID-19 transmission to include vaccination [13]. The model encapsulates the natural history of COVID-19 with classes of individuals including: susceptible; vaccinated; latently infected (not yet infectious); asymptomatic (and infectious); pre-symptomatic (and infectious); symptomatic with either mild or severe illness; recovered; and dead. Model population was stratified into six age groups of 0–4, 5–19, 20–49, 50–64, 65–79, and 80+ years based on US census data [14]. We sampled daily contacts within and between age groups from a negative-binomial distribution parameterized using an empirically-determined contact network (Appendix, Table A1) [15].
Disease dynamics
In our agent-based model, the risk of infection for people susceptible to COVID-19 depended on contact with infectious individuals that could be in asymptomatic, pre-symptomatic, or symptomatic stages of the disease. Using recent estimates, we parameterized the infectivity of asymptomatic, mild symptomatic, and severe symptomatic individuals to be 26%, 44%, and 89% relative to the pre-symptomatic stage [16–18]. For each infected individual, the incubation period was sampled from a Gamma distribution with a mean of 5.2 days [19]. A proportion of infected individuals developed symptomatic disease following a pre-symptomatic stage [20]. The duration of the pre-symptomatic stage and infectious period following symptom onset was sampled from a Gamma distribution with a mean of 2.3 days and 3.2 days, respectively [17,20,21]. Those who did not develop symptomatic disease remained asymptomatic until recovery, with an infectious period that was sampled from a Gamma distribution with a mean of 5 days [21,22]. We assumed that recovered individuals are immune against reinfection for the remainder of simulation timelines. Model parameters are summarized in Appendix, Table A2.
Infection outcomes
A proportion of severe symptomatic cases were hospitalized within 2–5 days of symptom onset [13,23], and thereafter did not contribute to the spread of infection. We assumed that all mild symptomatic cases and severely ill individuals self-isolated within 24 hours of symptom onset. The daily number of contacts during self-isolation was reduced by an average of 74%, based on a matrix derived from a representative sample population during COVID-19 lockdown [24]. Non-ICU and ICU admissions were parameterized based on age-stratified data for COVID-19 hospitalizations, and the presence of comorbidities [25,26]. The lengths of non-ICU and ICU stays were sampled from Gamma distributions with means of 12.4 and 14.4 days, respectively [27,28].
Vaccination
We implemented a two-dose vaccination campaign achieving 40% coverage of the entire population within one year. Prioritization was sequentially set to: (i) healthcare workers (5% of the total population) [29], adults with comorbidities, and those aged 65 and older; and (ii) other individuals aged 18–64 [30]. We assumed that 70% was the maximum achievable coverage in any age group, with an age-dependent distribution (Appendix, Table A3) mirroring seasonal influenza vaccination in the US [31]. We simulated a roll-out strategy offering 30 vaccine doses daily per 10,000 population, corresponding to 6.93 million vaccine doses per week for the entire US population. This rate corresponds to the goal of administering ~100 million vaccine doses in the first 100 days, as outlined by the Biden administration [32].
For Pfizer-BioNTech vaccines administered with no delay, the interval between the first and second doses was 21 days [2]. This interval was 28 days for Moderna vaccines [3]. Vaccine efficacy against symptomatic and severe disease was set to be 52% and 80% for vaccines from Pfizer-BioNTech and Moderna, respectively, 14 days after the first dose [2,3]. The corresponding efficacies following the second dose were 95% and 94%, respectively, 1 week after the second dose [2,3]. In the base case, we assumed that vaccine protection against infection was the same as efficacy against symptomatic disease after each dose of the vaccine. In a scenario analysis, we reduced the protection against infection to 50% of the efficacy against disease.
In order to evaluate the impact of vaccination with DSD relative to the tested schedules in clinical trials, we assumed that the estimated efficacies of the first and second doses of both Pfizer-BioNTech and Moderna vaccines would be maintained if the maximum time interval between doses did not exceed 6 weeks [33]. Starting from week 7, we applied a waning rate of 5% per week (against both infection and symptomatic disease). As sensitivity analysis, we also simulated the model without reduction of vaccine efficacy up to 18 weeks after the first dose. Further, we investigated a scenario in which the same reduction factor of 5% per week was also applied to the efficacy of the second dose, if the second dose was received later than 6 weeks after the first dose (Appendix, Figure A3). In this scenario, we assumed that with a sufficiently long delay between doses, administration of the second dose only restored the vaccine efficacy equivalent to that conferred by one dose (Appendix, Figures A4–A5).
Model scenarios
We considered a range of 5%−30% pre-existing immunity in the population, with 10% for the base-case scenario [34,35]. To parameterize the model at a given level of pre-existing immunity, we ran simulations in the absence of vaccination, and derived the infection rates in different age groups once the overall attack rate reached that pre-specified level. The corresponding age distributions of recovered individuals were used for the initial population at the start of vaccination. We simulated the model with a 10,000 population for a time horizon of one year to evaluate the impact of DSD vaccination relative to the recommended schedule on reducing attack rate, hospitalizations, and deaths for both Pfizer-BioNTech and Moderna vaccines. For results presented here, outcomes were averaged over 1000 independent replications of each scenario.
Results
DSD vaccination with waning efficacy of the first dose
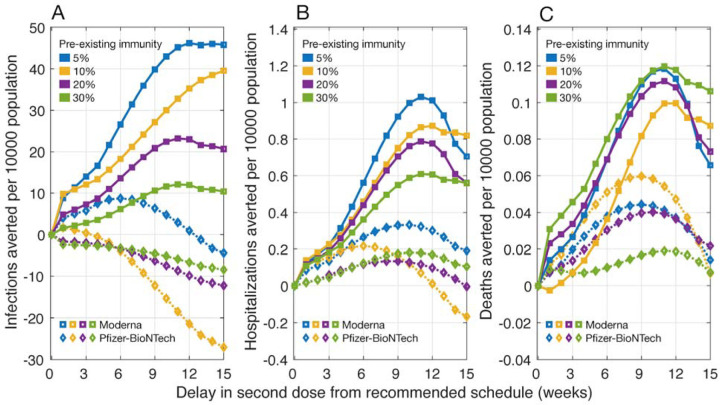
We found that delaying the second dose of the Moderna vaccines would lead to a larger reduction of infections, hospitalizations, and deaths, as compared to the recommended 28-day interval between doses (Figure 1). Hospitalizations and deaths can be minimized by delaying the second dose by 9–12 weeks. For example, at 10% pre-existing immunity and equal vaccine efficacy against infection and symptomatic disease, we projected that delaying the second dose of Moderna vaccines by 9–12 weeks would avert an additional 0.75 – 0.87 hospitalizations and 0.08 – 0.10 deaths per 10,000 population compared with the recommended vaccination schedule. This DSD schedule remains the most effective strategy across a range of pre-existing levels from 5% to 30% and even when vaccine efficacy against infection is 50% of the efficacy against symptomatic disease (Appendix, Figure A1).
Figure 1.
Projected number of infections, hospitalizations, and deaths averted per 10,000 population for a DSD vaccination program compared to the recommended schedule of two-doses of Pfizer-BioNTech (with a 21-day interval) and Moderna (with a 28-day interval) vaccines. The efficacy of vaccines in blocking infection transmission was assumed to be the same as the efficacy against symptomatic and severe disease. Waning of vaccine efficacy was 5% per week, starting from week 7 after the first dose. Efficacy after the second dose was 94% for Moderna and 95% for Pfizer-BioNTech vaccines.
For the Pfizer-BioNTech vaccines, we project that the DSD strategy would lead to an increase in the number of infections compared to the 21-day recommended schedule between doses in most scenarios of delay interval, pre-existing immunity levels and relative vaccine efficacy against infection and disease (Figure 1, Appendix: Figure A1). However, depending on the level of pre-existing immunity, DSD may avert more hospitalizations and deaths. These additional benefits are due to prioritization of elderly and individuals with comorbidities receiving the first dose; thus, reducing severe outcomes among these high-risk individuals.
DSD vaccination without waning efficacy of the first dose
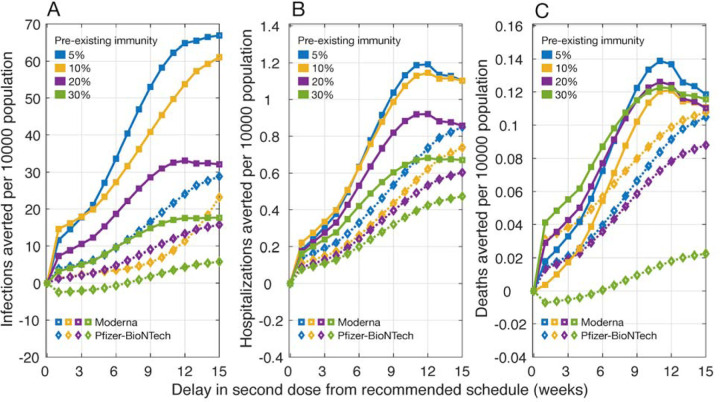
When the efficacy of the first dose did not wane for up to 18 weeks after being administered, we found that the DSD strategy averted more infections, hospitalizations, and deaths, compared to the recommended schedule for both Moderna and Pfizer-BioNTech vaccines (Figure 2). Similar to our results of waning vaccine efficacy, we found that delaying the second dose of Moderna vaccine by 9–12 weeks would lead to largest reductions in hospitalizations and deaths. At 10% pre-existing immunity, for example, such a strategy would avert 0.99 – 1.15 hospitalizations and 0.10 – 0.12 deaths per 10,000 population. The performance of DSD vaccination with Pfizer-BioNTech vaccines improved with an increasing delay of the second dose within 15 weeks from the recommended schedule. Although the scale of reduction varied, we observed a similar increasing trend for different levels of pre-existing immunity. Notably, DSD also outperformed the recommended vaccination schedule, even when there was a 50% reduction in the vaccine efficacy against infection compared to symptomatic disease (Appendix, Figure A2).
Figure 2.
Projected number of infections, hospitalizations, and deaths averted per 10,000 population for a DSD vaccination program compared to the recommended schedule of two-doses of Pfizer-BioNTech (with a 21-day interval) and Moderna (with a 28-day interval) vaccines. The efficacy of vaccines in blocking infection transmission was assumed to be the same as the efficacy against symptomatic and severe disease. There was no waning of vaccine efficacy within 15 weeks from the recommended schedule for the second dose. Efficacy after the second dose was 94% for Moderna and 95% for Pfizer-BioNTech vaccines.
DSD vaccination with waning efficacy of the first and second doses
Even with waning efficacy for both doses, DSD with Moderna vaccines would still lead to a larger reduction of infections, hospitalizations, and deaths, as compared to the recommended schedule, irrespective of whether vaccine efficacy against infection was the same or 50% lower than efficacy against disease (Appendix, Figures A4–A5). The lowest number of hospitalizations and deaths were achieved with a 9–12 week delay of the second dose. For Pfizer-BioNTech vaccines, we observed no clear advantage of DSD over the recommended schedule (Appendix, Figures A4–A5). Across the range of pre-existing immunity levels considered, DSD led to a higher number of infections. However, similar to the scenario of waning efficacy for only the first dose, DSD may avert more hospitalizations and deaths depending on the level of pre-existing immunity.
Discussion
Vaccination can have a substantial impact on mitigating COVID-19 outbreaks, even with limited protection against infection [36]. However, vaccine distribution in the US has not met the goal set by federal officials, with significant shortfalls in coverage and distribution since the start of vaccination on December 12, 2020 [37]. Challenges with vaccine supply and rollout, coupled with a deadly wave of outbreaks which has overwhelmed hospitals [38–40], and the emergence of highly transmissible SARS-CoV-2 variants [41–43], have sparked a debate as to whether available vaccines should be used to rapidly increase the coverage with the first dose, as a single-dose strategy in the near term [6–9]. While the US has committed to delivering the second dose on-time for those who receive the first dose [44], a few countries have approved guidelines for DSD, including Canada and the UK to defer the second dose by up to 6 and 12 weeks, respectively [45,46].
In this study, we evaluated whether deferral of the second dose beyond recommended schedules of 3 and 4 weeks for Pfizer-BioNTech and Moderna vaccines, respectively, could improve the effectiveness of vaccination programs in reducing attack rates, hospitalizations, and deaths. Under the assumption of waning efficacy for only the first dose over time, our results show that for Moderna vaccines, DSD vaccination with a delay of 9–12 weeks could enhance the program effectiveness by preventing additional infections and adverse outcomes, compared to a 4-week interval between the two doses (Figure 1). DSD with Pfizer-BioNTech vaccines beyond the 3-week tested schedule, on the other hand, may lead to a higher number of infections. However, depending on the level of pre-existing immunity, additional hospitalizations and deaths could be averted with DSD as a result of vaccine prioritization for individuals at higher risk of severe outcomes. We found that if the efficacy of the first dose did not wane until the administration of the second dose, then DSD for both Pfizer-BioNTech and Moderna vaccines is more effective than their recommended schedules (Figure 2).
Our findings highlight two important parameters in the evaluation of vaccination programs with DSD. First and foremost is the temporal waning of vaccine efficacy [11,12], which requires clinical and epidemiological studies monitoring vaccinated individuals for several weeks after inoculation with the first dose. Second is the ability of vaccines to block transmission which has not been quantified in the placebo-controlled efficacy data. In addition to these parameters, vaccine supply and many other factors such as the potential for emergence of vaccine-resistant strains under low individual-level protection; public confidence in vaccines; risk behaviour of individuals following vaccination; and the possibility of a drop in uptake of the second dose with a delay significantly longer than the recommended schedules, would be important considerations in public health decision-making regarding DSD vaccination [10]. Nevertheless, we found that even with sufficient vaccine supplies, the durability of vaccine efficacy and protection against infection still remain critical characteristics in determining the optimal time-interval between the two doses.
Supplementary Material
Funding.
Canadian Institutes of Health Research [OV4 – 170643, COVID-19 Rapid Research]; São Paulo Research Foundation [18/24811-1]; the National Institutes of Health [1RO1AI151176-01; 1K01AI141576-01], and the National Science Foundation [RAPID 2027755; CCF-1918784].
Footnotes
This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.
Reproducibility Statement. The computational system and parameters are available under an open source license at: https://github.com/thomasvilches/delayed_dose.
References
- 1.The New York Times. Coronavirus Vaccine Tracker. Available: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383: 2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2020; NEJMoa2035389. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Economist. Rich countries grab half of projected covid-19 vaccine supply. 2020. Available: https://www.economist.com/graphic-detail/2020/11/12/rich-countries-grab-half-of-projected-covid-19-vaccine-supply [Google Scholar]
- 5.SupplyChain247. The Complex Logistical Challenges of Vaccine Distribution. Jan 2021. Available: https://www.supplychain247.com/article/the_complex_logistical_challenges_of_vaccine_distribution [Google Scholar]
- 6.Tuite AR, Zhu L, Fisman DN, Salomon JA. Alternative Dose Allocation Strategies to Increase Benefits From Constrained COVID-19 Vaccine Supply. Ann Intern Med. 2021; M20–8137. doi: 10.7326/M20-8137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnabas RV, Wald A. A Public Health COVID-19 Vaccination Strategy to Maximize the Health Gains for Every Single Vaccine Dose. Ann Intern Med. 2021; M20–8060. doi: 10.7326/M20-8060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paltiel AD, Zheng A, Schwartz JL. Speed Versus Efficacy: Quantifying Potential Tradeoffs in COVID-19 Vaccine Deployment. Ann Intern Med. 2021; M20–7866. doi: 10.7326/M20-7866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matrajt L, Eaton J, Leung T, Dimitrov D, Schiffer JT, Swan DA, et al. Optimizing vaccine allocation for COVID-19 vaccines: critical role of single-dose vaccination. 2021. [cited 17 Jan 2021]. doi: 10.1101/2020.12.31.20249099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore John P.. How do you take your vacc ine—one lump or two? 2021. Available: https://blogs.bmj.com/bmj/2021/01/06/john-p-moore-how-do-you-take-your-vaccine-one-lump-or-two/ [Google Scholar]
- 11.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2021;384: 80–82. doi: 10.1056/NEJMc2032195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sewell HF, Robertson JF, Stewart M, Kendrick D, Bird SM. Revisiting the UK’s strategy for delaying the second dose of the Pfizer covid-19 vaccine. 2021. Available: https://blogs.bmj.com/bmj/2021/01/20/revisiting-the-uks-strategy-for-delaying-the-second-dose-of-the-pfizer-covid-19-vaccine/ [Google Scholar]
- 13.Shoukat A, Wells CR, Langley JM, Singer BH, Galvani AP, Moghadas SM. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. CMAJ Can Med Assoc J J Assoc Medicale Can. 2020;192: E489–E496. doi: 10.1503/cmaj.200457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Census Bureau QuickFacts U.S. Census Bureau QuickFacts. United States. Population Demographics. 2020. Available: https://www.census.gov/quickfacts/fact/table/US/PST045219 [Google Scholar]
- 15.Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, et al. Social Contacts and Mixing Patterns Relevant to the Spread of Infectious Diseases. Riley S, editor. PLoS Med. 2008;5: e74. doi: 10.1371/journal.pmed.0050074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368: eabb6936. doi: 10.1126/science.abb6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghadas SM, Fitzpatrick MC, Sah P, Pandey A, Shoukat A, Singer BH, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci. 2020;117: 17513–17515. doi: 10.1073/pnas.2008373117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayampanathan AA, Heng CS, Pin PH, Pang J, Leong TY, Lee VJ. Infectivity of asymptomatic versus symptomatic COVID-19. The Lancet. 2021;397: 93–94. doi: 10.1016/S0140-6736(20)32651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382: 1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26: 672–675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 21.Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020;368: 489–493. doi: 10.1126/science.abb3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatto M, Bertuzzo E, Mari L, Miccoli S, Carraro L, Casagrandi R, et al. Spread and dynamics of the COVID-19 epidemic in Italy: Effects of emergency containment measures. Proc Natl Acad Sci. 2020;117: 10484–10491. doi: 10.1073/pnas.2004978117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moghadas SM, Shoukat A, Fitzpatrick MC, Wells CR, Sah P, Pandey A, et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci U S A. 2020;117: 9122–9126. doi: 10.1073/pnas.2004064117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CMMID COVID-19 working group, Jarvis CI, Van Zandvoort K, Gimma A, Prem K, Klepac P, et al. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18: 124. doi: 10.1186/s12916-020-01597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69: 458–464. doi: 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC COVID-19 Response Team, CDC COVID-19 Response Team, Chow N, Fleming-Dutra K, Gierke R, Hall A, et al. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 — United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69: 382–386. doi: 10.15585/mmwr.mm6913e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8: 475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020;26: 1470–1477. doi: 10.3201/eid2607.200282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Bureau of Labor Statistics. Number of hospitals and hospital employment in each state in 2019 : The Economics Daily. Available: https://www.bls.gov/opub/ted/2020/number-of-hospitals-and-hospital-employment-in-eachstate-in-2019.htm [Google Scholar]
- 30.Committee on Equitable Allocation of Vaccine for the Novel Coronavirus, National Academy of Medicine, National Academies of Sciences, Engineering, and Medicine. Discussion Draft of the Preliminary Framework for Equitable Allocation of COVID-19 Vaccine. Washington, D.C.: National Academies Press; 2020. p. 25914. doi: 10.17226/25914 [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2018–19 Influenza Season. 2019. Available: https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm [Google Scholar]
- 32.CNN. Biden details plan to combat coronavirus pandemic in first 100 days. 11 Dec 2020. Available: https://www.cnn.com/2020/12/08/politics/biden-100-million-vaccines-100-days/index.html [Google Scholar]
- 33.World Health Organization. Interim recommendations for use of the Pfizer– BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing. 2021. Jan. Available: https://assets.documentcloud.org/documents/20445916/who-2019-ncov-vaccines-sage_recommendation-bnt162b2-20211-eng.pdf [Google Scholar]
- 34.SeroTracker. COVID-19 Seroprevalence. 2020. Dec. Available: https://serotracker.com/Dashboard [Google Scholar]
- 35.CDC COVID Data Tracker. United States COVID-19 Seroprevalence Estimate by States. Available: https://covid.cdc.gov/covid-data-tracker/#national-lab [Google Scholar]
- 36.Moghadas SM, Vilches TN, Zhang K, Wells CR, Shoukat A, Singer BH, et al. The impact of vaccination on COVID-19 outbreaks in the United States. medRxiv; 2020. Nov. doi: 10.1101/2020.11.27.20240051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NPR News. U.S. Likely Will Miss Goal Of Vaccinating 20 Million By The New Year. 31 Dec 2020. Available: https://www.npr.org/sections/coronavirus-live-updates/2020/12/31/952208601/u-s-likely-will-miss-goal-of-vaccinating-20-million-by-the-new-year [Google Scholar]
- 38.The Globe And Mail. Hospitals risk being overwhelmed because of holiday COVID-19 rule-breakers. 4 Jan 2021. Available: https://www.theglobeandmail.com/canada/article-hospitals-risk-being-swamped-because-of-holiday-rule-breakers/ [Google Scholar]
- 39.CTV News. Overwhelmed, California hospitals contemplate rationing care. 19 Dec 2020. Available: https://www.ctvnews.ca/health/coronavirus/overwhelmed-california-hospitals-contemplate-rationing-care-1.5238887 [Google Scholar]
- 40.CIDRAP. COVID-19 overwhelming hospitals, morgues in US, other nations. 10 Dec 2020. Available: https://www.cidrap.umn.edu/news-perspective/2020/12/covid-19-overwhelming-hospitals-morgues-us-other-nations [Google Scholar]
- 41.Mahase E. Covid-19: What new variants are emerging and how are they being investigated? BMJ. 2021; n158. doi: 10.1136/bmj.n158 [DOI] [PubMed] [Google Scholar]
- 42.Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. 2021. [cited 9 Jan 2021]. doi: 10.1101/2020.12.30.20249034 [DOI] [Google Scholar]
- 43.Centre for Mathematical Modelling of Infectious Diseases London School of Hygiene and Tropical Medicine. Estimated transmissibility and severity of novel SARS-CoV-2 Variant of Concern 202012/01 in England. Available: https://cmmid.github.io/topics/covid19/reports/uk-novel-variant/2020_12_23_Transmissibility_and_severity_of_VOC_202012_01_in_England.pdf [Google Scholar]
- 44.The Washington Post. U.S. health officials say they plan to stick with two-dose coronavirus regimen. 4 Jan 2021. Available: https://www.washingtonpost.com/health/2021/01/04/covid-vaccine-one-shot/ [Google Scholar]
- 45.Global News. Canada can delay 2nd coronavirus vaccine dose if there’s a shortage, panel says. 13 Jan 2021. Available: https://globalnews.ca/news/7573376/coronavirus-vaccine-2nd-dose-delay/ [Google Scholar]
- 46.The Guardian. Covid-19 second-stage vaccinations to be delayed across UK. 30 Dec 2020. Available: https://www.theguardian.com/world/2020/dec/30/covid-19-second-stage-nhs-vaccinations-delayed-across-uk [Google Scholar]
- 47.Systrom K, Vladek T, Krieger M. Rt.live. GitHub repository. 2020. Available: https://github.com/rtcovidlive/covid-model. Accessed 16 Nov 2020. [Google Scholar]
- 48.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25. doi: 10.2807/1560-7917.ES.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiura H, Kobayashi T, Miyama T, Suzuki A, Jung S, Hayashi K, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis. 2020;94: 154–155. doi: 10.1016/j.ijid.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimball A, Hatfield KM, Arons M, James A, Taylor J, Spicer K, et al. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility — King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69: 377–381. doi: 10.15585/mmwr.mm6913e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.