ABSTRACT
Background: We report the long-term outcomes, changes in laboratory parameters, the incidence of secondary nosocomial infections and treatment cost of a Spanish cohort of patients with severe COVID-19 that received tocilizumab (TCZ).
Methods: Retrospective cohort of PCR confirmed adult patients who received TCZ from March 1 to 24, 2020 in a tertiary hospital was analyzed. Patients were followed up until 10 May 2020.
Results: We included 162 patients (median age 64 years; 70.4% male). At time of TCZ administration, 48.1% of patients were on invasive mechanical ventilation (IMV). Over a median follow-up of 53 days, 46.9% of patients were discharge in good conditions and 19.8% were still hospitalized. The overall mortality was 33.3%, being higher in patients on IMV than those who did not (46.2% vs 26.7%, P < 0.001). A significant improvement in the lymphocyte count, C-reactive protein, lactate dehydrogenase, and D-dimer was observed. Overall, 43.2% patients presented nosocomial infections, causing death in 8%. Infections were more prevalent in ICU units (63.0% vs 17.1%, P < 0.001). The total cost of TCZ was €371,784.
Conclusions: Among the patients who used TCZ, one third died, regardless the improvement in some inflammatory biomarkers. The incidence of secondary nosocomial infections was high.
KEYWORDS: COVID-19, mortality, SARS-CoV-2, Spain, tocilizumab
1. Introduction
The clinical deterioration of SARS-CoV-2 infection would be the result of a combination of direct cytopathic effects induced by the virus and the immunopathology induced by a cytokine storm syndrome (CRS) associated with the immune response against to the virus. Results from a meta-analysis including 1,426 COVID-19 patients demonstrated that elevated IL-6 on admission was associated with an increased likelihood of mortality [1], and the inflammatory cytokine signature has been proved to predict COVID-19 severity and survival [2].
Tocilizumab (TCZ) is a recombinant humanized anti-IL-6 receptor monoclonal antibody that might block the hyperinflammation and CRS. During the first months of the COVID-19 pandemia, few small and short-term real-life cohorts with encouraging preliminary results prompted the off-label use of TCZ to reduce mortality [3–7]. However, the recent publication of preliminary results of four randomized trials did not show clear evidence of efficacy [8]. In addition, concerns exist regarding the increased incidence of secondary infections after TCZ administration [9]. This, combined with its shortage problems and high costs, makes it necessary to better identify its real benefits.
In this study, we report the long-term outcomes of a Spanish cohort of hospitalized patients with severe COVID-19 that received TCZ, including the changes in laboratory parameters and the incidence of secondary nosocomial infections. Treatment costs were also analyzed.
2. Patients and methods
2.1. Study design and participants
Ours was a single-center, observational, and retrospective study performed in a tertiary hospital, serving approximately a population of 350,000 inhabitants in Madrid, Spain. At the peak of the pandemic on the 24th of March, this hospital had 1,064 COVID beds, of which 134 were intensive care units (ICU) beds.
The study sample comprised all consecutive hospitalized adult patients (≥18 years) with confirmed SARS-CoV-2 infection who received at least one dose of TCZ from March 1 to 24, 2020. Patients were followed through 10 May 2020.
After patients provided informed verbal consent, they received standard and supportive care according to the hospital’s protocol for the management of COVID-19 (Appendix). Routine blood examinations included complete blood cell count, serum biochemical tests (renal and liver function profile, lactate dehydrogenase -LDH- and creatine kinase -CK-), C-reactive protein (CRP) and coagulation profile. When needed, procalcitonin, serum ferritin, interleukin-6 -IL-6- and myocardial enzymes (N-terminal pro-brain natriuretic peptide -NT-proBNP- and troponin) were performed. Chest radiographs or computerized tomography scan (CT) were also done when necessary.
The criteria for patient hospital discharge were lack of fever for at least 3 days, substantial improvement in both lungs and chest CT and clinical remission of respiratory symptoms.
The decision to initiate TCZ treatment was the responsibility of the treating physician following the recommendations of the Spanish Agency of Medicines and Medical Devices (AEMPS). In March 2020, the AEMPS recommended prioritizing the use of TCZ in patients who met all the following criteria: a) diagnosis of interstitial pneumonia and severe respiratory failure (defined as score = 2 in the COVID respiratory severity scale [10]) b) rapid respiratory worsening requiring noninvasive or invasive ventilation, c) severe systemic inflammatory response (high levels of IL-6 ->40 pg/mL-, or alternatively increased D-dimer ->1500 ng/mL- or progressive increase of D-dimer) and d) patients likely to be admitted to the ICU. Elevated serum levels of IL-6 were not a requirement for the initiation of TCZ, as long as elevated levels of D-dimer were present, due to the lack of availability of the IL-6 technique in Spanish hospitals during the first weeks of the pandemic.
The initial proposed dosing regimen of TCZ was a weight-based of 8 mg/kg to a maximum of 800 mg per dose and up to a maximum of 3 doses separated 12–24 hours based on patient clinical response. From March 19, after reviewing the available evidence and in response to shortage problems, TCZ was used at a fixed dose: a first dose of 600 mg followed by a second dose of 600 mg (in patients with body weight ≥ 80 kg) or 400 mg (if < 80 kg) 12 hours apart with the chance of assessing a third dose of 400 mg 16–24 hours after the second infusion if there were partial or incomplete clinical response.
TCZ doses were reconstituted by the Pharmacy Department with 100 mL 0.9% sodium chloride solution and administrated by intravenous infusion over 30–60 minutes.
2.2. Assessments
The following clinical variables were collected and analyzed during the course of the study: patient demographics, comorbidities, initial and subsequent laboratory tests, anti-COVID-19 treatment, either antiviral or immunosuppressive, oxygen therapy and clinical outcomes.
Clinical outcomes included discharge disposition, mortality, need for invasive mechanical ventilation (IMV), clinical improvement and length of hospital stay. Clinical improvement was evaluated on days 7, 14 and 28 after TCZ administration. Improvement was defined by live discharge from the hospital, a decrease of two points from baseline on a modified ordinal scale (as recommended by the WHO R&D Blueprint Group), or both. The six-point scale consists of the following categories: 1, not hospitalized; 2, hospitalized, not requiring supplemental oxygen; 3, hospitalized, requiring supplemental oxygen; 4, hospitalized, requiring nasal high-flow oxygen therapy, non-IMV, or both; 5, hospitalized, requiring IMV, extracorporeal membrane oxygenation (ECMO), or both; and 6, death.
Changes in the laboratory values of lymphocytes, IL-6, LDH, D-dimer, ferritin and CRP were followed and compared on days 7 and 14 after TCZ administration.
The incidence, attributable mortality, type and causative microorganisms of microbiologically documented nosocomial infections after TCZ treatment were recorded.
The cost of TCZ, as well as the rest of the immunosuppressive and antiviral agents for COVID-19, was calculated based on the dose administered and the official prices in Spain (including taxes).
2.3. Statistical analysis
Continuous variables were described by median and interquartile range (IQR) and categorical variables by frequencies and percentages. The Wilcoxon signed-rank test was used for comparisons between two continuous variables. The x2 test or Fisher`s Exact test was used to compare two categorical variables. Linear mixed models were used to estimate the effect of TCZ in the laboratory parameters. A P value <0.05 was considered statistically significant.
Computer support used for the statistical analysis was IBM SPSS Statistics 19® (SPSS Inc., Chicago, IL).
2.4. Ethical aspects
The study protocol was approved by the Ethics Committee of the hospital (FARM-COVID-19 v.1) and by the Spanish Agency of Medicines and Medical Devices (CGR-REM-2020-06), in accordance with the principles of the Declaration of Helsinki 2008.
3. Results
From March 1 to 24 March 0165 adult hospitalized patients received at least one dose of TCZ because of SARS-CoV-2 infection. Three patients were excluded due to a lack of laboratory-confirmation of COVID-19. A total of 162 patients were included in the study, which accounted for 14.0% of COVID-19 patients that required hospitalization during this period. The median follow-up time was 53 days (RIC 51–53).
3.1. Demographics and clinical features at admission
The median age of patients was 64 years (IQR 53–73) and most were male (70.4%) and Caucasian (84.0%). Overall, 30.9% were obese. The most common comorbidities were hypertension (45.1%), followed by cardiovascular disease (26.5%) and diabetes (25.9%) (Table 1).
Table 1.
Baseline demographic, clinical and treatment characteristics of the patients
| All patients (N = 162) |
Patients discharged alive and died (n = 130) |
P value | ||
|---|---|---|---|---|
| Discharge alive (n = 76) |
Death (n = 54) |
|||
| Demographics and presenting clinical features at admission | ||||
| Age | 64 (53–72) | 59 (48–69) | 71 (63–75) | <0.001 |
| >/ = 65 | 77 (47.5) | 26 (34.2) | 38 (70.4) | <0.001 |
| <65 | 85 (52.5) | 50 (65.8) | 16 (29.6) | <0.001 |
| Male | 114 (70.4) | 50 (65.8) | 42 (77.8) | 0.139 |
| Race | ||||
| Caucasian | 136 (84.0) | 65 (85.5) | 41 (75.9) | 0.164 |
| Latin American | 25 (15.4) | 11 (14.5) | 12 (22.2) | 0.254 |
| Asian | 1 (0.6) | 0 | 1 (1.9) | 0.234 |
| Current smoker | 10 (6.2) | 6 (7.9) | 4 (7.4) | 0.918 |
| Obesity (BMI >30) | 50 (30.9) | 16 (21.1) | 18 (33.3) | 0.116 |
| Comorbidities | ||||
| Hypertension | 73 (45.1) | 23 (30.3) | 33 (61.1) | <0.001 |
| Cardiovascular disease | 43 (26.5) | 14 (18.4) | 17 (31.5) | 0.085 |
| Diabetes | 42 (25.9) | 11 (14.5) | 20 (37.0) | 0.003 |
| Chronic lung disease | 10 (6.2) | 4 (5.3) | 5 (9.3) | 0.376 |
| Asthma | 10 (6.2) | 7 (9.2) | 0 | 0.022 |
| Chronic kidney disease | 20 (12.3) | 6 (7.9) | 8 (14.8) | 0.210 |
| Liver disease | 6 (3.7) | 4 (5.3) | 1 (1.9) | 0.319 |
| Tumor | 6 (3.7) | 4 (5.3) | 0 | 0.087 |
| HIV | 1 (0.6) | 0 | 1 (1.9) | 0.234 |
| Immunosuppressive therapy | 11 (6.8) | 5 (6.6) | 5 (9.3) | 0.572 |
| ACEi/ARB therapy | 57 (35.2) | 17 (22.4) | 28 (51.9) | <0.001 |
| Triage vitals | ||||
| Temperature, °C | 37.6 (36.9–38.3) | 37.6 (36.8–38.4) | 37.5 (37.1–38.0) | 0.609 |
| Temperature >37.3°C | 89 (59.3) | 45 (61.6) | 33 (67.3) | 0.966 |
| Pulse >/ = 125 beats per min | 5 (3.4) | 2/(2.8) | 1 (2.0) | 0.279 |
| Respiratory rate > 24 breath per min | 13 (61.9) | 4 (50.0) | 4 (80.0) | 0.782 |
| Systolic blood pressure < 90 mmHg | 1 (2.8) | 0 | 1 (2.2) | - |
| Oxygen saturation | ||||
| <94% | 99 (67.8) | 42 (60.0) | 35 (72.9) | 0.148 |
| <90% | 55 (37.7) | 19 (27.1) | 21 (43.8) | 0.061 |
| Laboratory findings at admission | ||||
| Hematologic | ||||
| Hemoglobin g/dL | 14.1 (13.2–15.4) | 14.2 (13.2–15.4) | 13.9 (12.4–15.1) | 0.187 |
| Hemoglobin <10 g/dL | 3 (1.9) | 0 | 2 (3.7) | 0.093 |
| White blood cells, x109/L | 6.2 (4.9–8.6) | 6.0 (4.9–7.6) | 6.9 (4.8–9.1) | 0.231 |
| <4x109/L | 45 (28.0) | 21 (28.0) | 16 (29.6) | 0.840 |
| >10x109/L | 18 (11.2) | 5 (6.7) | 8 (14.8) | 0.129 |
| Lymphocyte, x109/L | 0.8 (0.6–1.1) | 0.9 (0.6–1.2) | 0.8 (0.5–1.0) | 0.082 |
| <1x109/L | 103 (64.0) | 41 (54.7) | 37 (68.5) | 0.112 |
| Neutrophil, x109/L | 4.7 (3.5–6.8) | 4.2 (3.5–6.4) | 5.1 (3.6–7.7) | 0.192 |
| <1.5x109/L | 2 (1.2) | 1 (1.3) | 2 (1.6) | 0.814 |
| Platelets, x109/L | 168.0 (138.0–212.5) | 166.0 (136.0–219.0) | 169.5 (140.3–205.5) | 0.778 |
| <100x109/L | 11 (6.8) | 3 (4.0) | 6 (11.1) | 0.118 |
| Biochemical | ||||
| Creatinine, mg/dL | 0.92 (0.78–1.17) | 0.88 (0.73–1.02) | 1.01 (0.83–1.32) | 0.002 |
| >1.3 mg/dL | 26 (16.5) | 6 (8.1) | 15 (28.8) | <0.001 |
| Alanine aminotransferase, U/L | 38.0 (22.0–60.0) | 38.5 (21.3–63.8) | 37.0 (28.0–60.0) | 0.884 |
| >40 U/L | 67 (43.2) | 34 (47.2) | 20 (39.2) | 0.378 |
| Total bilirubin, mg/dL | 0.5 (0.4–0.7) | 0.5 (0.4–0.7) | 0.6 (0.4–0.7) | 0.349 |
| >1.1 mg/dL | 8 (5.6) | 3 (4.2) | 4 (8.7) | 0.319 |
| Lactate dehydrogenase, U/L | 343.0 (262.0–479.0) | 295.0 (244.0–415.0) | 443.0 (333.5–616.5) | 0.001 |
| >245 U/L | 75 (82.4) | 32 (74.4) | 27 (93.1) | 0.043 |
| Creatinine kinase, U/L | 128.5 (80.8–254.8) | 133.0 (71.0–264.0) | 121.0 (85.0–201.0) | 0.752 |
| >300 U/L | 23 (14.3) | 11 (23.4) | 6 (15.4) | 0.442 |
| Infection related indices | ||||
| C-reactive protein, mg/dL | 12.5 (5.7–18.4) | 7.9 (4.6–15.2) | 14.6 (8.6–21.1) | 0.002 |
| >0.5 mg/dL | 147 (100) | 68 (100.0) | 49 (100.0) | - |
| Procalcitonin, mcg/L | 0.13 (0.08–0.32) | 0.09 (0.06–0.16) | 0.17 (0.09–0.52) | <0.001 |
| >0.5 mcg/L | 21 (16.7) | 5 (8.5) | 10 (24.4) | 0.028 |
| Coagulation function | ||||
| Prothrombin time, s | 13.5 (12.7–14.3) | 13.3 (12.7–14.1) | 13.0 (12.7–14.1) | 0.681 |
| >13.5 s | 70 (40.7) | 30 (42.3) | 22 (44.0) | 0.848 |
| D-dimer, ng/mL | 304.0 (202.5–561.0) | 299.0 (188.0–497.0) | 534.0 (258.0–832.0) | 0.024 |
| >1,200 ng/mL | 11 (10.9) | 6 (12.8) | 3 (9.7) | 0.676 |
| Myocardial injury | ||||
| Troponin, pg/mL | 25.0 (11.0–135.5) | 11.0 (2.0–1,525.0) | 73.5 (16.3–155.3) | 0.217 |
| >34 pg/mL | 7 (41.2) | 1 (16.7) | 5 (62.5) | 0.086 |
| NT-proBNP, pg/mL | 432.0 (243.5–1,179.8) | 335.0 (86.0–1,119.0) | 458.5 (208.0–1,076.8) | 0.477 |
| >300 pg/mL | 33 (75.0) | 10 (66.7) | 16 (72.7) | 0.692 |
| Oxygen requirements at time of TCZ administration | ||||
| Low-flow oxygen | 8 (4.9) | 7 (9.2) | 1 (1.9) | 0.085 |
| Non- Invasive mechanical ventilation/High flow oxygen | 76 (46.9) | 48 (63.2) | 17 (31.4) | <0.001 |
| Invasive mechanical ventilation | 78 (48.1) | 21 (27.6) | 36 (66.7) | <0.001 |
| Medications | ||||
| Antiviral treatment | ||||
| HCQ + LPV/r + IFN-b | 86 (53.1) | 32 (42.1) | 38 (70.4) | 0.001 |
| HCQ + LPV/r + IFN-b + AZT | 31 (19.1) | 19 (25.2) | 8 (14.8) | 0.158 |
| HCQ + LPV/r | 24 (14.8) | 13 (17.1) | 5 (9.3) | 0.202 |
| HCQ + LPV/r + AZT | 19 (11.7) | 10 (13.2) | 3 (5.6) | 0.154 |
| Other | 2 (1.2) | 2 (2.6) | 0 | 0.230 |
| Remdesivir* | 31 (19.1) | 12 (15.8) | 11 (20.4) | 0.500 |
| Corticosteroid treatment** | ||||
| Corticosteroid | 122 (75.3) | 49 (64.5) | 44 (81.5) | 0.034 |
| With TCZ | 57 (35.2) | 31 (40.8) | 15 (27.8) | 0.005 |
| After TCZ | 65 (40.1) | 18 (23.7) | 29 (53.7) | 0.005 |
| Pulses | 29 (17.9) | 14 (18.4) | 7 (13.0) | 0.405 |
| Low-intermediate doses | 93 (57.4) | 35 (46.1) | 37 (68.5) | 0.011 |
| TCZ Treatment | ||||
| Time from symptom onset to TCZ administration | 10.0 (8.0–13.0) | 11.0 (8.8–13.0) | 9.5 (7.0–13.0) | 0.198 |
| Time of hospital admission to TCZ administration | 4.0 (2.0–7.0) | 4.0 (3.0–7.0) | 4.0 (2.0–6.0) | 0.191 |
| Nº of TCZ doses | ||||
| 1 dose | 60 (37.0) | 30 (39.5) | 17 (31.5) | 0.350 |
| 2 doses | 45 (27.8) | 25 (32.9) | 17 (31.5) | 0.865 |
| 3 doses | 57 (35.2) | 21 (27.6) | 20 (37.0) | 0.255 |
| Other treatments | ||||
| Concomitant antibiotic treatment | 157 (96.9) | 72 (94.7) | 53 (98.1) | 0.319 |
| LMWH | 153 (94.4) | 72 (94.7) | 51 (94.4) | 0.942 |
| Vasopressors | 90 (55.6) | 23 (30.3) | 42 (77.8) | <0.001 |
Data are presented as median (IQR) or n (%). For clinical studies and laboratory testing for which not all patients had values, percentages of total patients with completed tests are shown.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AZT, azithromycin; BMI, body mass index; HCQ, hydroxychloroquine; IFN-b, β-interferon 1b; LMWH, low-molecular-weight heparin; LPV/r, lopinavir/ritonavir; NT-proBNP, N-terminal pro-brain natriuretic peptide; RDV, remdesivir; TCZ, tocilizumab.
*All patients were treated with an alternative antiviral therapy until remdesivir was available.
**Corticosteroid treatment was classified as pulse dose if ≥ 125 mg of methylprednisolone or equivalent was administered every 24 h, or as low-intermediate dosage otherwise.
Time from the onset of symptoms to hospital admission was 5.9 (IQR 4.0–7.0) days. At admission, 59.3% of patients were febrile and 67.8% had an arterial oxygen saturation value below 94%. Baseline laboratory findings are also summarized in Table 1. The most remarkable abnormal findings include the following: elevated LDH (82.4%), lymphocytopenia (64.0%), elevated alanine aminotransferase (43.2%), elevated CK (14.3%) and D-dimer (10.9%). At admission, all patients presented elevated high sensitivity C-reactive protein (CRP), whereas only 16.7% presented elevated procalcitonine.
Overall, 98.8% of patients had bilateral infiltrates on the chest radiography.
All patients received initially an off-label treatment with antiviral agents, being the most frequent combination lopinavir/ritonavir (LPV/r) plus hydroxychloroquine plus β-interferon 1b (Table 1). The majority of patients also started with concomitant antibiotic therapy until bacterial coinfection was discarded. All patients in the ICU were evaluated by an infectious diseases specialist that provided antibiotic stewardship recommendations.
3.2. Patient characteristics at the time of TCZ administration
The median of days from admission to TCZ administration was 4.0 days (IQR 2.0–7.0). At this time, all patients were receiving supplemental oxygen, 56.8% were admitted in the ICU and 48.1% were on IMV. Notable laboratory findings at the moment of TCZ administration include median D-dimer of 1,032 mcg/mL (IQR 485–2,763), ferritin of 1,343 mcg/L (IQR 881–2,749), CRP of 16.6 mg/dL (IQR 9.6–26.6) and peripheral lymphocyte count of 0.6 × 109/L (IQR 0.4–1.1) (Table 2). Baseline value of IL-6 was available in 11% of patients, with a median of 98.5 pg/mL (IQR 50.0–153.0).
Table 2.
Laboratory findings
| Day of TCZ administration | + 7 days | + 14 days | |
|---|---|---|---|
| Hematologic | |||
| Hemoglobin g/dL | 12.8 (11.7–13.8) | 12.0 (11.0–14.0) | 11.5 (9.8–12.6) |
| Hemoglobin < 10 g/dL | 12 (8.1) | 127 (87.6) | 30 (27.0) |
| White blood cells, x109/L | 7.9 (5.8–11.0) | 10.5 (6.5–13.6) | 9.9 (5.9–15.7) |
| <4x109/L | 28 (18.8) | 22 (15.2) | 18 (16.2) |
| >10x109/L | 50 (33.6) | 79 (54.5) | 55 (49.5) |
| Lymphocyte, x109/L | 0.6 (0.4–0.9) | 0.8 (0.5–1.3) | 1.0 (0.7–1.6) |
| <1x109/L | 117 (78.5) | 78 (53.8) | 53 (47.7) |
| Neutrophil, x109/L | 6.7 (4.8–9.8) | 8.5 (4.8–12.0) | 8.2 (4.0–13.6) |
| <1.5x109/L | 1 (0.7) | 4 (2.8) | 6 (5.4) |
| Platelets, x109/L | 209.0 (162.5–287.0) | 248.0 (173.5–324.0) | 165.0 (124.0–227.0) |
| <100x109/L | 9 (6.0) | 10 (6.9) | 13 (11.7) |
| Biochemical | |||
| Creatinine, mg/dL | 0.82 (0.64–1.17) | 0.74 (0.56–1.20) | 0.66 (0.49–0.98) |
| >1.3 mg/dL | 19 (13.2) | 29 (20.4) | 21 (20.0) |
| Alanine aminotransferase, U/L | 45.0 (31.8–69.0) | 73.0 (43.5–128.5) | 64.0 (42.0–119.0) |
| >40 U/L | 81 (55.5) | 113 (77.9) | 83 (76.9) |
| Total bilirubin, mg/dL | 0.9 (0.6–1.4) | 0.7 (0.4–1.2) | 0.7 (0.5–1.1) |
| >1.1 mg/dL | 51 (35.4) | 39 (28.3) | 83 (78.3) |
| Lactate dehydrogenase, U/L | 451.0 (369.8–584.3) | 368.0 (289.0–463.0) | 350.5 (278.8–449.3) |
| >245 U/L | 129 (99.2) | 103 (88.0) | 66 (86.8) |
| Creatinine kinase, U/L | 145.5 (77.8–323.0) | 77.0 (44.0–196.0) | 79.5 (40.8–159.3) |
| >300 U/L | 21 (17.3) | 21 (16.9) | 8 (8.3) |
| Infection related indices | |||
| C-reactive protein, mg/dL | 16.6 (9.6–26.6) | 0.5 (0.4–1.4) | 0.4 (0.4–1.2) |
| >0.5 mg/dL | 127 (96.9) | 66 (48.2) | 30 (31.3) |
| Procalcitonin, mcg/L | 0.17 (0.09–0.86) | 0.04 (0.02–0.13) | 0.02 (0.05–0.13) |
| >0.5 mcg/L | 32 (27.8) | 10 (9.6) | 8 (9.9) |
| Ferritin, mcg/L | 1,343.5 (881.5–2,749.3) | 1,231.0 (680.0–2,168.5) | 917.0 (641.0–1,395.0) |
| >200 mcg/L | 22 (100) | 13 (100) | 15 (100) |
| IL-6, pg/mL | 98.5 (50.0–153.0) | 342.0 (36.0–1,718.5) | 64.0 (35.0–112.5) |
| >40 pg/mL | 18 (90.0) | 4 (80.0) | 2 (50.0) |
| Coagulation function | |||
| Prothrombin time, s | 14.2 (13.2–15.7) | 12.2 (11.7–13.0) | 12.3 (11.6–13.2) |
| >13.5 s | 146 (100) | 17 (12.5) | 18 (17.0) |
| D-dimer, mcg/mL | 1,032.0 (485.0–2,763.5) | 1,949.0 (708.5–4,684.0) | 1,103.0 (524.0–2,879.0) |
| >1,200 mcg/mL | 49 (45.0) | 56 (57.7) | 45 (49.5) |
| Myocardial injury | |||
| Troponin, pg/mL | 15.0 (7.0–28.0) | 7.0 (2.0–20.0) | 4.0 (2.0–21.5) |
| >34 pg/mL | 8 (18.6) | 6 (19.4) | 4 (20.0) |
| NT-proBNP, pg/mL | 127.0 (99.0–172.0) | 646.0 (219.0–1,841.0) | 181.5 (78.5–1,949.5) |
| >300 pg/mL | 41 (82.0) | 21 (67.7) | 8 (44.4) |
Data are presented as median (IQR) or n (%). For laboratory testing for which not all patients had values, percentages of total patients with completed tests are shown.
Abbreviations: NT-proBNP, N-terminal pro-brain natriuretic peptide.
The majority of patients (63.0%) received two or three dosages of TCZ. Overall, 75.3% of patients also received corticosteroids, 35.2% in combination with TCZ and 40.1% after TCZ administration.
3.3. Outcomes
At the end of the follow-up, 80.2% of patients completed their hospital course and 19.8% continued hospitalized. Overall, 46.9% of patients were discharged alive, with a median length of hospital stay of 23.5 days (IQR 16.8–35.0). The mortality rate was 33.3% (Figure 1A). Of patients who completed their hospital course, 41.5% died. Of these, 68.5% had received two or three doses of TCZ, with a median time from first TCZ administration to death of 14.0 days (IRQ 4.0–30.0).
Figure 1.
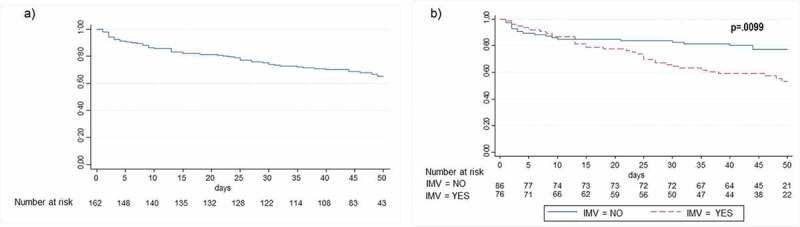
Kaplan-Meier survival estimates of the cumulative probability of death in the total population (1A) and in patients who received TCZ under IMV or not (1B)
Abbreviations: IMV, invasive mechanical ventilation; TCZ, tocilizumab
Patients who died were more frequently old, male and suffered from hypertension and diabetes in comparison to survivors. Likewise, the values at admission of creatinine, LDH, CRP, procalcitonine and D-dimer were significantly higher in non-survivors (Table 1).
Mortality rate was higher in those who received TCZ under IMV (46.2% vs 21.4%, HR 2.0 -IC95% 1.2–3.5- P = 0.010) (Figure 1B). Of 84 patients who received TCZ without IMV, 22.6% needed a subsequent intubation in a median of 2.0 days (IQR 1.0–7.0) after TCZ treatment.
A sub-analysis stratified by the presence of elevated serum levels of IL-6 prior to TCZ administration (18 patients) showed a mortality rate at the end of the follow-up of 11.1%.
3.3.1. Changes in oxygen-support
Figure 2 shows the changes in oxygen-support status of patients on days 7, 14 and 28 post-TCZ treatment. An improvement was observed in five patients (3.1%), 46 (28.4%), and 72 (44.4%) on days 7, 14, and 28, respectively. In a subgroup of patients without IMV, 59.2% showed an improvement in the ordinal scale on day 28, compared to 26.9% of those receiving IMV.
Figure 2.
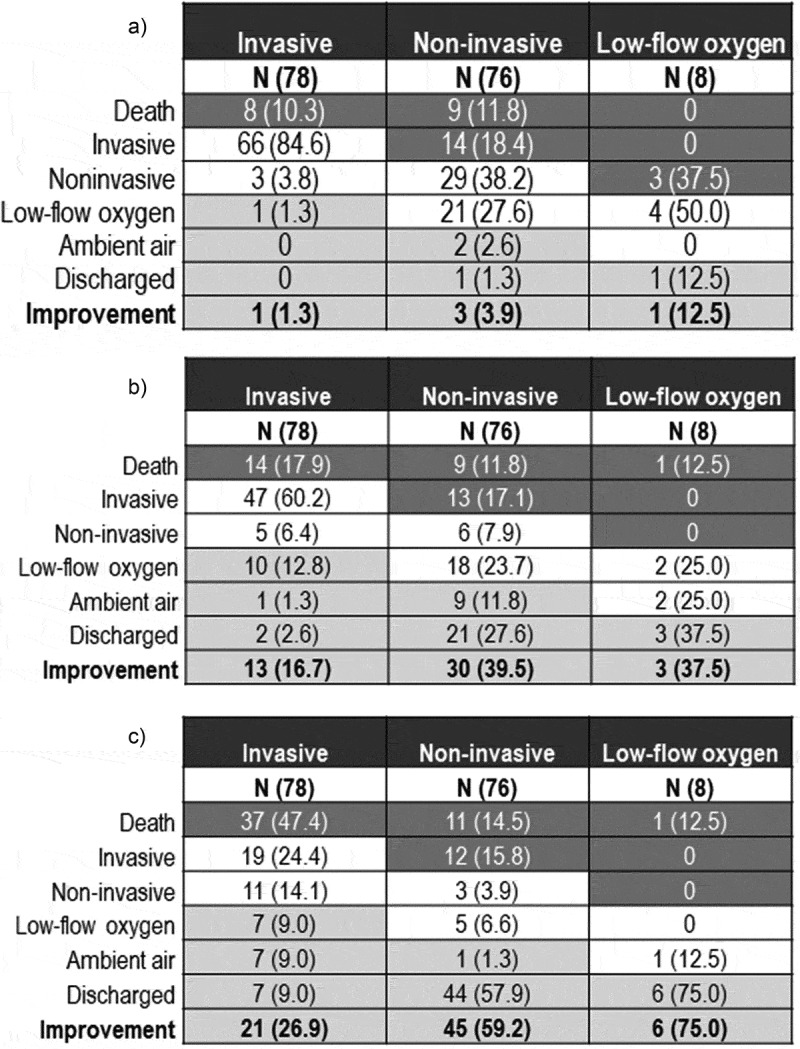
Oxygen-support status at baseline and after TCZ treatment
Figure 2A) Oxygen-Support Status at Baseline and 7 days after TCZ treatment. Figure 2B) Oxygen-Support Status at Baseline and 14 days after TCZ treatment. Figure 2C) Oxygen-Support Status at Baseline and 28 days after TCZ treatment.For each oxygen-support category, percentages were calculated with the number of patients at baseline as the denominator. Improvement (light gray cells), no change (beige) and worsening (dark gray) in oxygen-support status are shown. Noninvasive ventilation includes nasal high-flow oxygen therapy, noninvasive positive pressure ventilation, or both. Abbreviations: TCZ: tocilizumab.
In the subgroup of 18 patients in whom the presence of elevated IL-6 levels could be confirmed at the time of TCZ administration, the rate of clinical improvement was 38.1% and 52.4% on days 14 and 28, respectively. No improvement was observed on day 7.
3.3.2. Changes in laboratory values
Changes in laboratory parameters on days 7 and 14 post TCZ treatment are showed in Table 2. The values of CRP and procalcitonin decreased and returned to normal ranges in 90.1% and 68.8% of patients, respectively. In the case of D-dimer, 9% of patients had normal values on day +14. Not significant change was observed for ferritin, which remained elevated in all patients. On the contrary, a transitory increase in IL-6 was observed on day +7, from 98.5 pg/mL to 342.0 pg/mL, and a subsequent decrease to 64.0 pg/mL (IQR 35.0–112.5) on day +14. However, this is explained by the mechanism of action of TCZ, which inhibits the IL-6 receptor resulting in an increased level of unbound IL-6.
A summary of laboratory parameters throughout the entire follow-up period is shown in Figure 3. A gradual increase in the lymphocyte count and a decrease in the rest of the parameters was observed during hospitalization, with the exception of ferritin. It is important to note the significant drop in CRP and D-dimer levels after TCZ treatment, reaching greatest decline between days +7 and +14.
Figure 3.
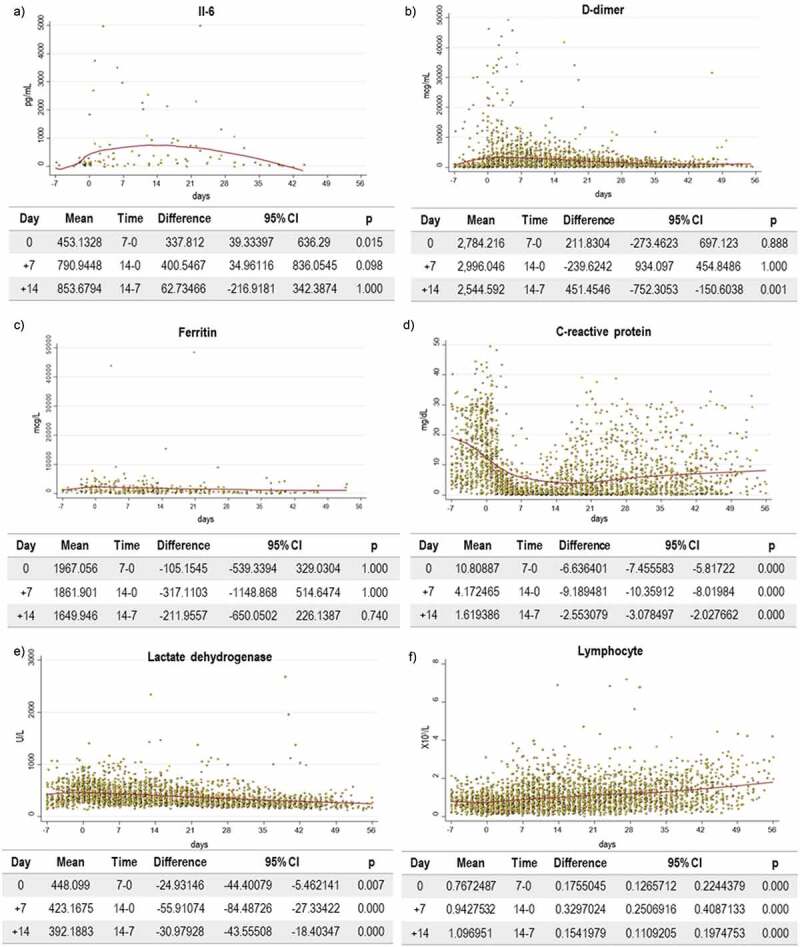
Categorical summary of laboratory parameter after TCZ treatment
Figure 3A) Categorical summary of IL-6 after TCZ treatment. Figure 3B) Categorical summary of D-dimer after TCZ treatment. Figure 3C) Categorical summary of ferritin after TCZ treatment. Figure 3D) Categorical summary of C-reactive protein after TCZ treatment. Figure 3E) Categorical summary of lactate dehydrogenase after TCZ treatment. Figure 3F) Categorical summary of lymphocyte after TCZ treatment. Abbreviations: CI: confidence interval; TCZ: tocilizumab.
3.3.3. Incidence of nosocomial infections
Overall, 70 (43.2%) patients had 147 microbiologically demonstrated nosocomial infections (Table 3). Of them, 26.5% acquired more than one infection. The incidence of nosocomial infections was higher in ICU patients (63.0% vs 17.1%, P <0.001). The main episodes of infection were ventilator associated pneumonia (25.3%), catheter-related bacterial bloodstream infection (16.0%), urinary tract infection (16.0%) and non- catheter-related bacterial bloodstream infection (13.0%).
Table 3.
Secondary infections
| Overall (n = 162 patients) |
ICU (n = 92 patients) |
No ICU (n = 70 patients) |
|
|---|---|---|---|
| Nº Patients with secondary infection | 70 (43.2) | 58 (63.0) | 12 (17.1) |
| Nº of secondary infections | 147 | 126 | 21 |
| Infection per patient | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 1.0 (1.0–2.3) |
| Type of secondary infection | |||
| Ventilator associated pneumonia | 41 (25.3) | 36 (39.1) | 5 (7.1) |
| CR-BSI | 26 (16.0) | 22 (23.9) | 4 (5.7) |
| Urinary tract infection | 26 (16.0) | 24 (26.1) | 2 (2.8) |
| Bacteremia other than CR-BSI | 21 (13.0) | 17 (18.5) | 4 (5.7) |
| Cytomegalovirus reactivation or disease | 12 (7.4) | 9 (4.7) | 3 (4.3) |
| Skin and soft tissue infection | 7 (4.3) | 6 (6.5) | 1 (1.4) |
| CR- Candida bloodstream infection | 6 (3.7) | 5 (5.4) | 1 (1.4) |
| Hospital-acquired pneumonia | 4 (2.5) | 3 (3.3) | 1 (1.4) |
| C. difficile infection | 2 (1.2) | 2 (2.2) | 0 |
| Gastrointestinal not C. difficile | 2 (1.2) | 2 (2.2) | 0 |
Data are presented as n (%) or median (IQR).
Abbreviations: CR, catheter-related; CR-BSI, catheter-related bacterial bloodstream infection; ICU, Intensive Care Unit.
Microbiological agents isolated from significant clinical samples are described in Table 4. The most frequent were coagulase negative Staphylococcus (10.8%), Pseudomonas aeruginosa (10.2%) and Enterococcus faecium (9.6%). Only 10/167 (6.0%) of the bacterial infections were produced by multidrug resistant (MDR) bacteria (6 Klebsiella pneumoniae -extended spectrum betalactamase and carbapenemase producers-, 3 methicillin-resistant Staphylococcus aureus and 1 MDR P. aeruginosa).
Table 4.
Microbiological agents isolated from clinical samples
| N (%) | |
|---|---|
| Total | 167 (100) |
| Coagulase negative Staphylococcus | 18 (10.8) |
| Pseudomonas aeruginosa | 17 (10.2) |
| P. aeruginosa MDR | 1 (0.6) |
| Enterococcus faecium | 16 (9.6) |
| Staphylococcus aureus methicillin- susceptible | 15 (9.0) |
| Staphylococcus aureus methicillin- resistant | 3 (1.8) |
| Enterobacter sp. | 13 (7.8) |
| Cytomegalovirus reactivation | 12 (7.2) |
| Candida sp. | 11 (6.6) |
| Enterococcus faecalis | 9 (5.4) |
| Escherichia coli | 8 (4.8) |
| Klebsiella | 7 (4.2) |
| Klebsiella ESBL/CRE | 6 (3.6) |
| Non-fermenting gram negative bacillus | 6 (3.6) |
| Aspergillus sp. | 5 (3.0) |
| Burkholderia | 4 (2.4) |
| Citrobacter | 4 (2.4) |
| Other Streptococcus sp. | 3 (1.8) |
| Aerobic gram-positive bacillus | 3 (1.8) |
| Serratia sp. | 3 (1.8) |
| Morganella | 3 (1.8) |
| C. difficile | 2 (1.2) |
| Proteous sp. | 2 (1.2) |
| Strenotrophomonas | 2 (1.2) |
| Streptococcus pneumoniae | 1 (0.6) |
| Other anaerobes | 1 (0.6) |
| Other yeast | 1 (0.6) |
| Other enteropathogens | 1 (0.6) |
Data are presented as n (%).
Abbreviations: CRE: carbapenem resistant enterobaccteriaceae, ESBL: extended spectrum betalactamase; MDR: multidrug resistant.
Death was directly attributable to the infection in 13 (18.6%) cases, of whom 11 were in ICU.
3.4. Treatment costs
The total economic impact of anti-COVID-19 agents, either antiviral or immunosuppressive, was €399,404 (€2,215 per patient, IQR 1,244–3,222). The total cost of TCZ treatment was €371,784, which accounted for 93.1% of the total cost. The median TCZ costs per-patient was €2,096 (IQR 1,048–3,143). The total cost of antivirals was €26,497 (€151 per patient, IQR 78–228). The highest expense in antivirals was due to the consumption of β-interferon 1b (€15,668), followed by LPV/r (€9,857). Remdesivir (RDV) was purchased at no cost through the Compassionate Use Access Program, which was enabled through the collaboration of the provider (Gilead Sciences, Inc.) and the AEMPS. The cost of steroids was 1,123 (€7 per patient, IQR 4–10).
4. Discussion
We reported the off-label use of TCZ in a cohort of 162 COVID-19 patients admitted to one of the Spanish hospitals most severely affected by the pandemic. To our knowledge, this is the first cohort that details long-term outcomes, including the changes in laboratory parameters and the incidence of secondary nosocomial infections.
The hyperinflammatory state observed in some critically ill COVID-19 patients led to an urgent and widespread use of TCZ during the first month of the pandemic, despite lack of data from randomized clinical trials. In Spain, TCZ was prioritized for very severe COVID-19 patients that fulfilled the criteria defined by the Spanish Agency of Medicines and Medical Devices (Appendix). In this study, 14.0% of hospitalized patients met these criteria and received TCZ. Our population was generally elderly, with an elevated prevalence of comorbid conditions and with a poor respiratory status at admission. At the time of TCZ administration (on average, 4 days from admission), their laboratory values were indicative of a rapid deterioration with an impaired immune-inflammatory profile, characterized by lymphocytopenia and elevated CRP, ferritin, LDH, and D-dimer. Up to 57% of patients were hospitalized in an ICU and 48% were on IMV at the time of TCZ administration.
Our high prevalence of critical COVID-19 patients can explain, in part, a higher mortality rate (33%) compared to other previous reports that describe the use of TCZ (7–20%) [3–5,11,12]. However, these cohorts are not directly comparable, not only because of their lower prevalence of seriously ill patients (3–43%) but also because they were very small, heterogenous, and had a very short period of follow-up (7–14 days), insufficient to accurately assess mortality after TCZ administration. In addition, they presented lower proportion of patients with advanced age and underlying conditions such as hypertension and diabetes, which have been strongly associated with poorer outcomes [13,14]. On the contrary, two recent Spanish studies in patients without IMV have shown low mortality rates (2–10%), associating TCZ treatment with a reduction in ICU admissions, intubation or death in this subpopulation [15,16].
Of particular concern is our high mortality rate in patients who received TCZ under IMV (46% vs 21% of patients on non-IMV). Morena et al. found that the mortality rate was significantly associated with IMV at baseline (83% vs 20%, P <0.001) in a cohort of 51 patients that were followed a median of 34 days after TCZ administration [17]. Somers et al. found that TCZ was effective in reducing mortality in an observational controlled study of 154 patients under mechanical ventilation (18% vs 36% in the standard care group), with a 45% reduction in hazard of death (hazard ratio 0.55 − 95% CI 0.33, 0.90-) [18]. However, the propensity score and regression analyses of observational datasets have important limitations, first and foremost that unmeasured confounding variables may still be present.
On the other hand, newly released randomized trials do not show clear evidence of efficacy [8]. Preliminary results of the largest trial (COVACTA) pointed out that TCZ did not improve the clinical status in patients with severe COVID-19 associated pneumonia, nor did it reduce mortality at day 28 (TCZ = 19.7% vs placebo = 19.4%). However, it is important to note that its eligibility criteria were broad, patients were in different stages of the disease and the results were not stratified by clinical signs of hyperinflammation. Pending the results from other clinical trials, a greater efficacy cannot be ruled out if TCZ is used before acute respiratory failure, presumably at this earlier stage of the disease the benefits of the drug could be greater.
Of note, our mortality rate was lower in a small subgroup of patients with baseline elevated serum levels of IL-6 (11%). This raises the question: Should TCZ be employed in case elevated IL-6 values cannot be confirmed, regardless of other inflammatory biomarkers? A recent retrospective cohort of 146 patients in a Spanish hospital showed that increased levels of IL-6 predict IMV requirement in patients with severe disease and contribute to establish an adequate indication for TCZ administration [19]. Another crucial question concerns the relative utility of TCZ treatment versus other nonspecific immunomodulatory agents, including corticosteroids. In our study, 75% of patients received corticosteroids, and we were unable to determine if these could have enhanced the effects of TCZ.
Regarding laboratory parameters, we observed a gradual increase in the lymphocyte count and a decrease in inflammatory biomarkers after TCZ treatment, which is consistent with previous studies [4–7,10,14]. In our patients, this effect was especially notable in the CRP levels, which is a likely reflection of TCZ’s immune modulating effect [20]. Specifically, with IL-6, we observed a significant increase on the first days after TCZ treatment, with a non-significant reduction on day +14.
Another interesting finding is the high prevalence of nosocomial infections in our population (43%), an issue that remains poorly described. Overall, 33% of patients developed ventilator-associated bacterial pneumonia or bloodstream infection, and 8% died because of a superinfection. The prevalence of nosocomial infections in ICU units (63%) was significantly higher compared to that found in our ICU population that did not receive TCZ during the same period (39%, P = 0.057). Quartuccio et al. found the same prevalence of nosocomial infections in patients treated with TCZ (43%), which increased up to 71% in ICU patients [21]. Similarly, Somers et al. found an incidence of 54% in patients under mechanical ventilation [18]. Therefore, these results highlight the need to strengthen supplementary stewardship strategies, including educational interventions for personnel with limited experience, for the treatment of future COVID-19 patients.
Finally, the cost of TCZ treatment is also a concern, as it accounts for 93.1% of expenditure in anti-COVID-19 agents. This increase in cost is particularly important when compared with the cost of corticosteroids.
Our study is subject to the limitations inherent to observational studies. First, there was no randomization or control group. Second, the concomitant use of corticosteroids represents an evident confounding factor in the analysis of potential therapeutic efficacy. Lastly, due to the retrospective study design, not all laboratory tests were done in all patients, including lactate dehydrogenase, IL-6, and serum ferritin. However, this study represents one of the longest cohorts of patients treated with TCZ reported to date, with a long period of follow-up. It also provides consistent data about the changes on different inflammatory biomarkers.
5. Conclusions
Our study shows a mortality rate of 33% in severe COVID-19 patients who receive TCZ, despite the improvement in some inflammatory biomarkers. The incidence of secondary nosocomial infections was high. Data from ongoing randomized, controlled clinical trials will be crucial to determine if a greater benefit can be achieved with earlier administration of TCZ and better selection of candidate patients.
Acknowledgments
The authors thank Athento® for their assistance in data mining. The authors thank J.M. Bellón for his assistance with the statistical analyses. The authors thank Angelica Minero Escobar for editing the article.
Appendix.
Appendix. Antiviral and immunosuppressive combinations for COVID-19 recommended in the protocol of the hospital during the study period.
| Setting | Clinical characteristics | Treatment* |
|---|---|---|
| Outpatients | Respiratory infection without pneumonia | Observation |
| Respiratory infection without pneumonia but with the presence of comorbidities | Observation and individualize treatment | |
| Non-severe pneumonia in patients without comorbidities | LPV/r + HCQ*. If contraindication: HCQ + AZT** | |
| Inpatients | Non-severe pneumonia in patients <65 years old and without comorbidities | LPV/r + HCQ*. If contraindication: HCQ + AZT** |
| Severe pneumonia, patients >65 years old or with comorbidities | LPV/r + HCQ + interferon β 1b*. If contraindication: HCQ + AZT** Assess the use of corticosteroids (dexamethasone or metylprednisolone) from day +8 after symptoms onset if increased need for oxygen support, chest X-ray with involvement of two or more lobes, increased D-dimer, C-reactive protein, lactate dehydrogenase or ferritin. |
|
| If worsening and/or criteria of systemic inflammatory response |
|
AZT, azithromycin; HCQ, hydroxychloroquine; LPV/r, lopinavir/ritonavir; RDV, remdesivir.
*Dosages recommended for each drug:
LPV/r: 400/100 mg every 12 h orally
HCQ: 400 mg/12 h orally x 2 dosages, followed by 200 mg/12 h
AZT: 500 mg, followed by 250 mg/24 h orally
RDV: 200 mg, followed by 100 mg/24 h intravenously
Dexamethasone: 20 mg/24 h as an intravenous bolus during 5 days, followed by 10 mg/24 h during another 5 days
Methylprednisolone 1–2 mg/kg/24 h as an intravenous bolus during 3–5 days
Tocilizumab: before 19 March , 8 mg/kg to a maximum of 800 mg per dose and up to a maximum of 3 doses separated 12–24 hours based on patient clinical response; after 19 March, weight ≥80 kg: 600 mg intravenously, followed by a second dose of 600 mg (12 h apart); weight <80 kg: 600 mg intravenously, followed by a second dose of 400 mg (12 h apart). Assess the need for a third administration of 400 mg at 16–24 h after the second infusion if partial or incomplete clinical response.
**Serial electrocardiograms are recommended (baseline and every 48 h).
Funding Statement
This work was partially supported by a grant from MSD [Innovando Juntos initiative, in cooperation with the University Carlos III of Madrid, the University of Seville, the Spanish Society of Infectious Diseases and Clinical Microbiology, the Spanish Society of Hospital Pharmacy, and the Association of Science Parks and Technological of Spain].
Article highlights
In the beginning of the pandemic tocilizumab use was not guided by IL6 serum levels
The overall mortality was 33% (46% with mechanical ventilation vs 27% without)
An improvement in inflammatory parameters was observed, except for ferritin
The incidence of nosocomial infections was 43%, causing death in 8% of patients
The cost of tocilizumab was high (median cost per patient €2,096).
Author contributions
E. Chamorro-de-Vega and C. Rodriguez-Gonzalez drafted the manuscript, critically revised the manuscript for important intellectual content. S. Manrique-Rodríguez substantially contributed to conception or design, contributed to acquisition, analysis, or interpretation of data. E. Lobato-Matilla, F. Garcia-Moreno, P. Ruiz-Briones, R. Romero-Jiménez, A. Gimenez-Manzorro, R. Collado-Borrell, J.L. Revuelta-Herrero, B. Somoza-Fernandez, S. Garcia-Sanchez, C. Sarobe-González, C.M. Fernandez-Llamazares and I. Taladriz-Sender contributed to acquisition, analysis, or interpretation of data. M. Olmedo, R. Correa-Rocha, T. Aldámiz-Echevarria, M. Valerio, M. Machado, M. Sancho-Gonzalez and M. Sancho-Gonzalez critically revised the manuscript for important intellectual content. E. Bouza, A. Herranz, P. Muñoz and M. Sanjurjo substantially contributed to conception or design.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Aziz M, Fatima R, Assaly R.. Elevated interleukin‐6 and severe COVID‐ 19: a meta‐analysis. J Med Virol. 2020;92(11):2283–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Valle DM, Kim-Schulze S, Huang H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020. [Published Online 2020 June24];2(8):e474–e484. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sciascia S, Aprà F, Baffa A, et al. Pilot prospective open, single-arm multicentre study on offlabel use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38(3):529–532. [PubMed] [Google Scholar]
- 6.Xu X, Hanb M, Lia T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alattar R, Ibrahim T, Shaar S, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020;92(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tleyjeh IM, Kashour Z, Damlaj M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clinical Microbiology and Infection: the Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020. November. DOI: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed]; •• Meta-analysis of efficacy and safety of tocilizumab that shows that tocilizumab reduces the risk of mechanical ventilation in hospitalized but did not reduce short-term mortality.
- 9.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duca A, Piva S, Focà E, et al. Calculated decisions: Brescia-COVID Respiratory Severity Scale (BCRSS)/algorithm. Emerg Med Pract. 2020. April 16; 22(5Suppl). CD1-CD2.PMID: 32297727 [PubMed] [Google Scholar]
- 11.Fernández-Ruiz M, López-Medrano F, Pérez-Jacoiste Asín MA, et al. Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: a single-center cohort study. Med Virol. 2020;1–12. DOI: 10.1002/jmv.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;12(323):1775–1776. [DOI] [PubMed] [Google Scholar]
- 14.CDC COVID‐19 Response Team . Severe outcomes among patients with coronavirus disease 2019 (COVID‐19)—United States. MMWR Morb Mortal Wkly Rep. 2020;69(12):343‐346. 10.15585/mmwr.mm6912e2. February12–March16, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno-Garcia E, Rico-Caballero V, Albiach L, et al. Tocilizumab is associated with reduction of the risk of ICU admission and mortality in patients with SARS-CoV-2 infection. 2020.. medRxiv. 10.1101/2020.06.05.20113738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Baño J, Pachón J, Carratalà J, et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAMCOVID-19). Clin Microbiol Infect. 2020;7(20):30492–30494. S1198-743X. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Multicentre study that suggest that tocilizumab might be useful in COVID-19 patients with a hyperinflammatory state.
- 17.Morena V, Milazzoa L, Orenia L, et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020:ciaa954. DOI: 10.1101/2020.05.29.20117358 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Cohort of mechanically ventilated COVID-19 patients in which tocilizumab was associated with lower mortality
- 19.Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J Allergy Clin Immunol. 2020. September 30; S0091-6749(20):31329–4. DOI: 10.1016/j.jaci.2020.09.018. Epub ahead of print. PMID: 33010257; PMCID: PMC7525244. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Study that concluded that baseline IL-6 levels greater than 30 pc/mL contributed to establish an adequate indication for TCZ administration.
- 20.Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quartuccio L, Sonagliaa A, McGonagle D, et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. 2020;129:104444. [DOI] [PMC free article] [PubMed] [Google Scholar]


