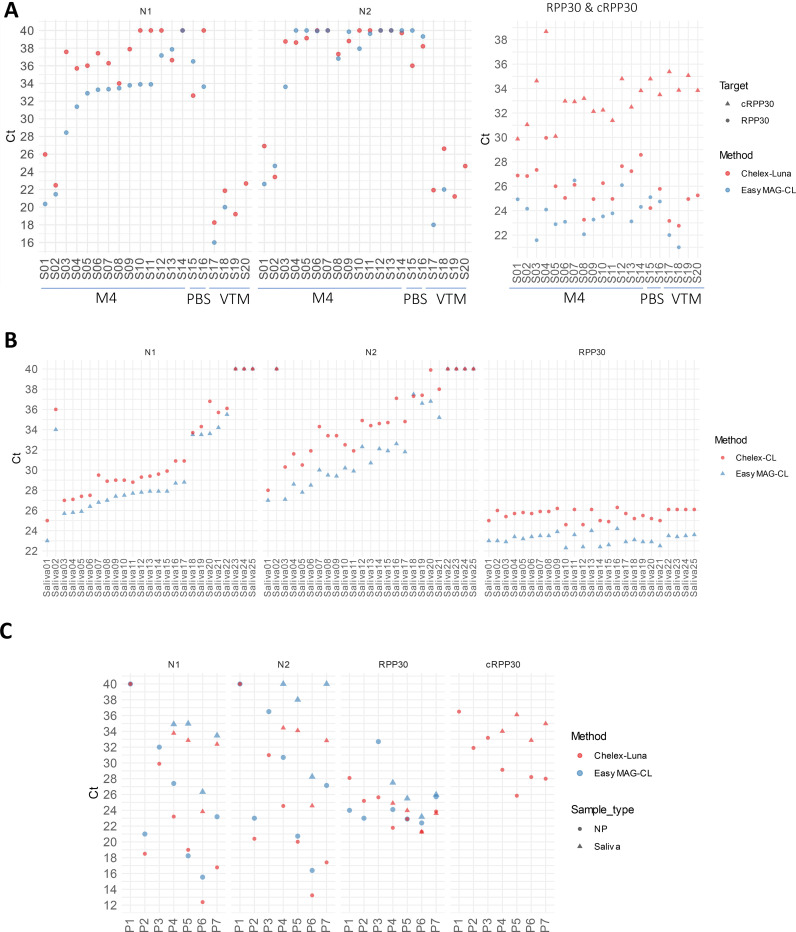
Figure 3.
SARS-CoV-2 detection of patient samples prepared by the Chelex method. (A) Patient NP swab samples were heated in the presence of 5% Chelex (S01 to S16, and S19, S20) or 10% Chelex (S17 & S18). S19 & S20 are 1:2 dilution of S17 & S18 in LowTE, respectively. (B) 50 μl of patient saliva samples (Saliva01 & 02) or negative patient saliva samples spiked with positive patient saliva samples (Salia03 to 22) were mixed with 25 μl of 50% Chelex in TED99, and heated for 5 min in a ThermoMixer. (C) Paired NP swabs from seven patients (P1 to P7) and saliva-saturated swabs from four patients (P4 to P7) were collected in VTM or Chelex collection tubes. VTM samples were used for RNA extraction (EasyMag). Luna refers to the NEB Luna RT-qPCR kit and NEB-Luna-Program II with 2.5 μl samples in a 10 μl reaction volume. CL refers to CDC assay performed in the clinical laboratory with 5 μl samples in a 20 μl reaction volume. Undetermined Ct values were plotted as Ct 40.