Fig. 3.
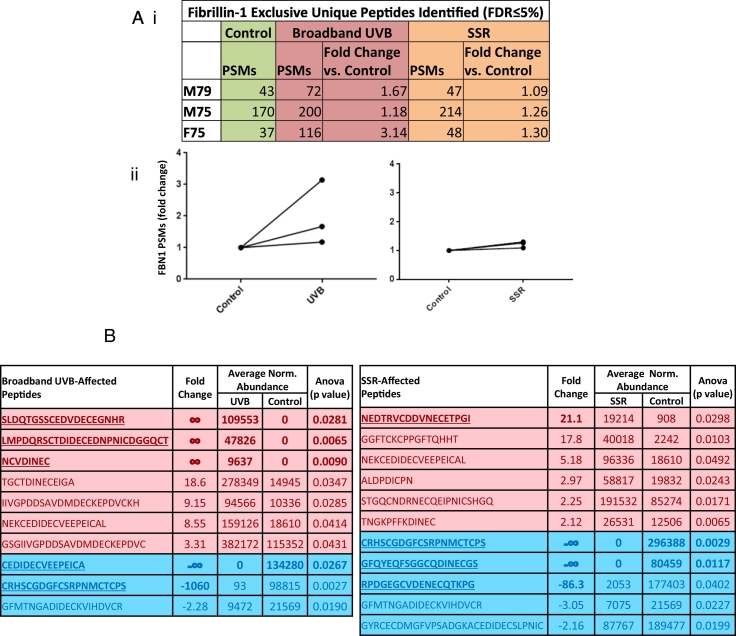
UV irradiation of HDF-derived microfibril isolations led to an increase in the overall proteolytic susceptibility of fibrillin-1. PSMs for broadband UVB- and SSR-irradiated fibrillin-1 per individual and their fold changes in comparison to control fibrillin-1 are shown (Peptide Prophet FDR ≤ 5%) (Ai). Broadband UVB irradiation led to an increase in fibrillin-1 peptide identification (2.00 average fold change) (Aii, left panel). Similarly, SSR irradiation of fibrillin-1 led to a smaller but consistent increase in fibrillin-1 peptide identification (1.22 average fold change) for all three replicates (Aii, right panel). Data-dependent peptide quantification revealed significant changes in the relative abundance of fibrillin-1 peptide sequences post-UVR exposure. Only peptides with fold changes greater than or equal to two were considered (B; N = 3). Ten fibrillin-1 peptides in broadband UVB-irradiated microfibril samples and eleven in SSR-irradiated samples were significantly increased (red) or decreased (blue) in relative abundance compared to control samples. Peptide sequences, fold changes relative to the control group, average normalised abundances and p values are shown (Progenesis multivariate paired ANOVA). Peptide sequences with fold changes greater than twenty are in bold and underlined.