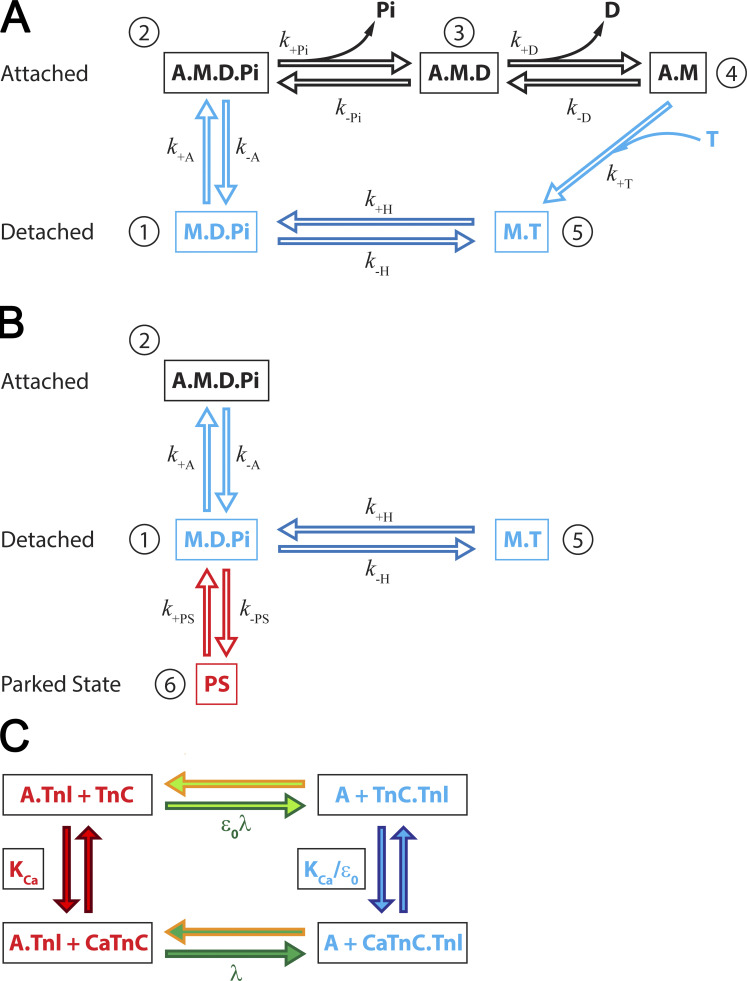
Figure 2.
Model of the cross-bridge cycle. (A) Five-state model of the actomyosin cycle includes biochemical states consistent with observed structural states: a detached state 1, M.D.Pi; weakly bound state 2, A.M.D.Pi; strongly bound post–power stroke state 3, A.M.D; rigor-like state 4, A.M; and detached state 5, M.T. The strain-dependent state transition rates are also associated with conformational changes defining the structural conformations of myosin in each state (i.e., power stroke, associated with Pi release, and stroke, δ, associated with the ADP release, ATP binding and cross-bridge detachment, and hydrolysis and reverse stroke). (B) Addition of a state representing interaction of myosin heads with the thick filament backbone, the so-called “parked state”, PS, denoted as state 6, into a five-state model from A. The PS is a partial M.D.Pi state with structural conformation associated with thick-filament backbone, reducing the population of the M.D.Pi state capable of binding to actin and therefore fluxes from M.D.Pi state to A.M.D.Pi state (myosin binding to actin) or the reverse hydrolysis to M.T state. The transition rate from PS to M.D.Pi, is assumed to be strongly dependent on [Ca2+], and the transition rate from M.D.Pi state to PS, is independent on [Ca2+]. (C) Kinetic scheme of calcium binding to TnC and interaction of TnI with actin in cardiac muscle. Calcium binding to TnC, with the equilibrium rate forms a Ca2+.TnC complex and reduces affinity of TnI to actin. The detachment rate of TnI from actin is defined by the equilibrium rate λ forming the CaTnC.TnI state. In CaTnC state, Tpm is free to move, mostly azimuthally, permitting myosin binding and force generation. The dissociation from A.TnI to TnC.TnI state without bound Ca2+ is slow, attenuated by and the calcium binding to TnC.TnI into the CaTnC.TnI complex is accelerated by a factor