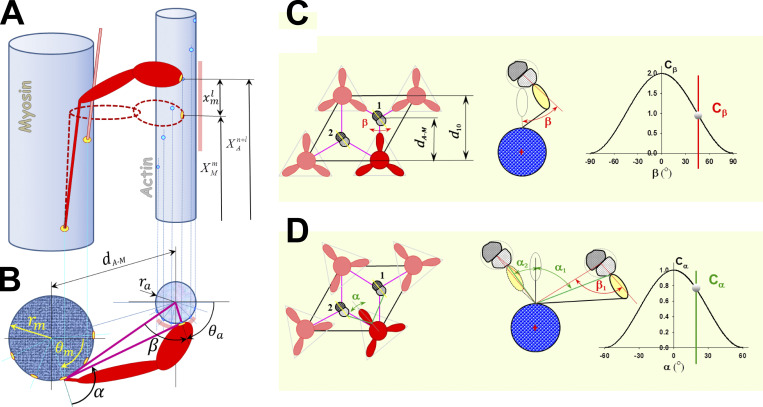
Figure S1.
Interaction between myosin heads and actin filaments in 3-D is defined by the triple-helical arrangement of myosin molecules along the myosin filament and the double-helical arrangement of monomers (myosin-binding sites) along the actin filaments. The 3-D geometry of myosin head binding domains and binding sites on actin in a sarcomere requires both longitudinal position matching and angular matching in the azimuthal plane. (A) Each myosin head m can move from its undeformed position along actin filament due to thermal agitation and can reach a few neighboring binding sites on actin. The myosin-binding domain is shown as a yellow oval at the tip of the myosin head, and binding sites on actin are shown as bright blue circles. The range of axial movement is shown as a pale red bar. The relative axial position of a myosin head (cross-bridge) and adjacent actin sites where superscripts and denote the index of an adjacent site on actin, and is the index of accessible sites on actin in neighborhood of m, respectively. The maximum number of adjacent sites on actin reachable by a myosin head m is denoted as La. To bind the site +l, the cross-bridge, including S2 and a myosin head, needs to stretch or compress axially for displacement (B) In the 3-D sarcomere lattice, the actin and myosin filaments are separated by spacing and sites on the actin filament (strand) are at an azimuthal angle β. In addition, a cross-bridge needs to turn from its equilibrium position by an angle α to reach an actin filament that is not aligned with its equilibrium angular position. For precise calculations of the angles, it is necessary to know the myosin equilibrium angular positions angular position of site on actin filament and diameters of myosin and actin filaments and respectively. The angular range of movement is denoted as a pale red arc around the actin filament. The azimuthal weight factors Cβ and Cα of myosin binding in 3-D sarcomere lattice are defined as in C and D. (C) When myosin heads in crown 1 are directly aligned with three actin filaments, then Cα = 1 and Cβ weights the azimuthal departure of a myosin-binding site on the actin filament from the plane passing through myosin and actin longitudinal axes, where the angle β is a function of the axial departure from perfect matching, ξ, which resembles the preference for myosin heads to bind to favorably oriented sites on the actin filament. (D) When myosin heads are not directly aligned with the surrounding actin filaments, such as with crowns 2 and 3, the weight factor Cα takes into account the departure by the angle α from perfect alignment between the heads on the crown and the reachable actin filaments. Figure S1 is adapted from Mijailovich et al. (2016).