Abstract
BACKGROUND
Preoperative pulmonary function plays an important role in selecting surgical candidates and assessing postoperative complications. Reduced pulmonary function is associated with poor survival in several cancers, but the prognostic value of preoperative pulmonary function in esophageal squamous cell carcinoma (ESCC) is unclear. Nutritional and systemic inflammation parameters are vital to cancer survival, and the combination of these parameters improves the prognostic value. The hemoglobin, albumin, lymphocytes and platelets (HALP) score is a novel prognostic indicator to reflect the nutritional and inflammation status, but the clinical effects of the HALP score combined with maximal voluntary ventilation (MVV), an important parameter of pulmonary function, have not been well studied in ESCC.
AIM
To investigate the prognostic value of MVV and HALP score for assessing postoperative survival of ESCC patients.
METHODS
Data from 834 ESCC patients who underwent radical esophagectomy with R0 resection were collected and retrospectively analyzed. Preoperative MVV and HALP data were retrieved from medical archives. The HALP score was calculated by the formula: Hemoglobin (g/L) × albumin (g/L) × lymphocytes (/L)/platelets (/L). The optimal cut-off values of MVV and HALP score were calculated by the receiver operating characteristic curve analysis. The Kaplan-Meier method with log-rank test was used to draw the survival curves for the variables tested. Multivariate Cox proportional hazard regression models were used to analyze the independent prognostic factors for overall survival.
RESULTS
MVV was significantly associated with gender (P < 0.001), age at diagnosis (P < 0.001), smoking history (P < 0.001), drinking history (P < 0.001), tumor length (P = 0.013), tumor location (P = 0.037) and treatment type (P = 0.001). The HALP score was notably associated with gender (P < 0.001), age at diagnosis (P = 0.035), tumor length (P < 0.001) and invasion depth (P = 0.001). Univariate Cox regression analysis showed that low MVV and low HALP score were associated with worse overall survival (all P < 0.001). Multivariate analysis showed that low MVV and the HALP score were both independent risk factors for overall survival (all P < 0.001). The combination of MVV and HALP score improved the prediction performance for overall survival than tumor-node-metastasis. Also, low combination of MVV and HALP score was an independent risk factor for poor overall survival (P < 0.001).
CONCLUSION
MVV, HALP score and their combination are simple and promising clinical markers to predict overall survival of ESCC patients.
Keywords: Maximal voluntary ventilation; Hemoglobin, albumin, lymphocytes and platelets score; Nutritional status; Inflammation status; Postoperative survival; Esophageal squamous cell carcinoma
Core Tip: Reduced pulmonary function is considered a risk factor for cancer survival. The combination score of hemoglobin, albumin, lymphocytes and platelets (HALP) is a novel prognostic indicator to reflect nutritional and inflammatory status. We demonstrated that preoperative maximal voluntary ventilation (MVV), an important parameter of pulmonary function, and HALP score were independent prognostic factors for patients with esophageal squamous cell carcinoma. The combination of MVV and HALP score has a better prognostic value than tumor-node-metastasis alone. The combination of MVV and HALP score reflects the status of inflammation, nutrition and pulmonary function simultaneously and may partly compensate for the limitation of the tumor-node-metastasis staging system.
INTRODUCTION
Esophageal cancer is the sixth leading cause of cancer-related death in the world[1]. Esophageal squamous cell carcinoma (ESCC) is the predominant type of esophageal cancer in China. Despite improvements in treatment and clinical management, the prognosis of ESCC is still poor[1]. Surgery remains the preferred method for non-metastatic cancer patients. Although the tumor-node-metastasis (TNM) staging system is well known as a predictive clinical parameter in terms of guiding treatment and clinical prognosis[2], the survival outcomes for esophageal cancer patients with the same TNM stage still vary widely. In addition, accurate TNM stage identification relies on the pathologic result after esophagectomy. Therefore, it is important to identify more effective preoperative clinical factors for early guidance of treatment and for predicting the prognosis of esophageal cancer, particularly in ESCC.
Preoperative pulmonary evaluation is a routine examination used to select potential surgical candidates and assess postoperative respiratory complications[3]. Previous studies have shown that poor pulmonary function is associated with severe postoperative complications in patients undergoing esophagectomy[4-6]. Some studies have reported that reduced pulmonary function is associated with mortality risk in the general population[7,8], lung cancer[9] and gastric cancer[10], while little attention has been given to the correlation between preoperative pulmonary evaluation and the overall survival of esophageal cancer patients. Forced vital capacity (FVC) and vital capacity (VC) are both important parameters in pulmonary evaluation test. Low FVC has been implicated as a risk prognostic predictor in gastric cancer[10], and low VC also predicts the poor survival in Japanese esophageal cancer[11] in previous limited studies. Maximal voluntary ventilation (MVV) is another important parameter in pulmonary evaluation test and is usually used to reflect respiratory muscle strength and lung capacity. Studies report that MVV reflects dysfunction and airway resistance both in inspiratory and expiratory phases, while FVC and VC reflect only the expiratory phase[12], so MVV may better reflects the real capacity of lung function. However, few studies have examined its effects on esophageal cancer patient survival.
Dysphagia is a typical symptom of esophageal cancer patients and may lead to malnutrition. Numerous studies have demonstrated that the parameters of nutrition and inflammation status, including the levels of hemoglobin and albumin and lymphocyte and platelet counts, are vital to cancer survival[13-16]. More importantly, some studies reported that the combination of these parameters such as neutrophil-lymphocyte ratio[17], platelet-to-lymphocyte ratio[18] and prognostic nutritional index[19] could better predict prognosis than nutritional or inflammatory condition status alone. The combination of hemoglobin, albumin, lymphocytes and platelets (HALP) score with four hematological parameters has been demonstrated as a novel and potential prognostic indicator for several types of malignancies[20-22], but the clinical effects of HALP score combined with other prognostic factors in ESCC patients who underwent esophagectomy have not been well studied.
Thus, to address these issues, we investigated the prognostic value of preoperative pulmonary function (MVV) and HALP score for the long-term survival in this group of ESCC patients.
MATERIALS AND METHODS
Patients
A total of 834 ESCC patients who underwent radical esophagectomy with R0 resection between 2011 and 2014 from the 500000 esophageal and gastric cardia carcinoma database (1973-2020), established by Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, were enrolled in the retrospective study. Patients were selected according to the following criteria: (1) Patients were diagnosed with ESCC by postoperative histopathology; (2) Patients had tumors located in the thoracic esophagus; (3) Patients had no other malignant tumors except for ESCC; (4) Patients received no chemotherapy or/and radiotherapy before surgery; (5) Patients had no neuromuscular disorders, respiratory diseases, chronic/acute inflammatory disease or autoimmune disease or not received glucocorticoid therapy before surgery; and (6) Preoperative records of pulmonary evaluation test and HALP parameters were obtained.
Clinical data collection and follow-up
The MVV value was determined and collected from routine preoperative pulmonary evaluation, and the values for HALP parameters were obtained from routine blood test before surgery. The HALP score was calculated using the following formula: Hemoglobin (g/L) × albumin (g/L) × lymphocytes (/L)/platelets (/L). The pathological TNM stage of patients was also collected on the basis of the 7th edition of the TNM staging system from the American Joint Committee on Cancer[2]. The time of diagnosis was the date when patients were confirmed to have ESCC by histopathology. The overall survival time was the interval from the time of diagnosis to death or the last follow-up. The last follow-up was performed in May 2020. Of the total 834 patients, 799 patients (95.8%) had detailed follow-up data. In the first year after esophagectomy, patients were followed up every 3 mo. Then, follow-up was conducted once a year.
Statistical analysis
SPSS software (version 21, IBM, Armonk, NY, United States) and GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, United States) were used to perform all the statistical analyses. Two-tailed P values of less than 0.05 were considered statistically significant. The optimal cut-off values of MVV and HALP score were calculated by the receiver operating characteristic (ROC) curve analysis. The chi-square test was used to analyze the relationship of clinical features with MVV and HALP score for patients with ESCC. A univariate Cox proportional hazard regression model was used to evaluate the prognostic value of each variable for overall survival. The Kaplan-Meier method with log-rank test was used to draw the survival curves for the variables tested. Multivariate Cox proportional hazard regression models were used to analyze the independent prognostic factors for overall survival. Significant prognostic variables in the univariate analysis were chosen for the multivariate analysis.
RESULTS
Patients’ basic clinical characteristics
From the archived clinical record, we retrieved the baseline clinic parameters for this group of ESCC patients (Table 1). The ratio of males to females was 1.6:1. The median age was 60 years, ranging from 38 to 84 years. Overall, 756 patients (90.6%) underwent open esophagectomy by the left approach, and 78 patients (9.4%) underwent surgery by the right approach, including 75 patients (9.0%) who were treated with open esophagectomy and three patients (0.4%) who were treated with thoracoscopic esophagectomy. 151 patients (18.1%) had postoperative complications. The median survival time of the ESCC patients was 4.4 years, ranging from 0.19 to 9.5 years. The 1-, 3- and 5-year survival rates of all patients were 91.4%, 64.6% and 42.9%, respectively.
Table 1.
Clinical characteristics of patients with esophageal squamous cell carcinoma
| Variable |
Cases
|
|
|
n
|
%
|
|
| Gender | ||
| Male | 509 | 61.0 |
| Female | 325 | 39.0 |
| Age at diagnosis in yr | ||
| Median (interquartile range) | 60 (55-65) | |
| Smoking history | ||
| No | 434 | 52.0 |
| Yes | 400 | 48.0 |
| Drinking history | ||
| No | 574 | 68.8 |
| Yes | 260 | 31.2 |
| Tumor length (cm) | ||
| Median (interquartile range) | 4.0 (3.0-5.0) | |
| Tumor location | ||
| Upper | 140 | 16.8 |
| Middle | 549 | 65.8 |
| Lower | 145 | 17.4 |
| Differentiation | ||
| Well | 37 | 4.5 |
| Moderate | 459 | 55.0 |
| Poor | 338 | 40.5 |
| Invasion depth | ||
| pT1 | 108 | 12.9 |
| pT2 | 240 | 28.8 |
| pT3 | 486 | 58.3 |
| Lymph node metastasis | ||
| pN0 | 494 | 59.2 |
| pN1 | 190 | 22.8 |
| pN2 | 121 | 14.5 |
| pN3 | 29 | 3.5 |
| TNM stage | ||
| I | 100 | 12.0 |
| II | 454 | 54.4 |
| III | 280 | 33.6 |
| Surgical approach | ||
| Left | 756 | 90.6 |
| Right | 78 | 9.4 |
| Treatment type | ||
| Surgery | 650 | 77.9 |
| Surgery + adjuvant RT/CT | 184 | 22.1 |
| MVV | ||
| Median (interquartile range) | 85.1 (68.5-96.6) | |
| HALP score | ||
| Median (interquartile range) | 43.2 (31.6-55.4) | |
| Overall complications | ||
| No | 683 | 81.9 |
| Yes | 151 | 18.1 |
CT: Chemotherapy; HALP: Hemoglobin, albumin, lymphocytes and platelets; MVV: Maximal voluntary ventilation; RT: Radiotherapy; TNM: Tumor-node-metastasis.
Association of MVV and HALP score with basic clinical characteristics
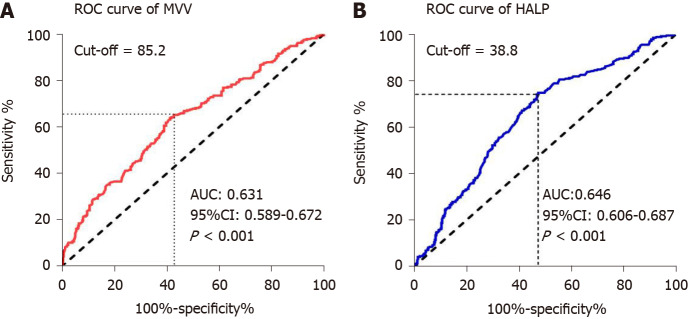
To assess the potential correlation of MVV and HALP score for overall survival, we first performed ROC analysis using their values in these ESCC patients. We found that the area under the curve (AUC) of MVV and HALP score were 0.631 and 0.646, respectively, indicating that they were both significant for predicting 5-year overall survival (all P < 0.001, Figure 1). Then, we determined the optimal cutoff values of MVV and HALP score as 85.2 and 38.8, respectively, by using the maximal Youden index. Based on the two cutoff values, the ESCC patients were divided into high and low groups for both MVV and HALP score, respectively. We then applied the chi square test to evaluate the potential association of MVV and HALP score with basic clinical factors. We found that MVV was significantly associated with gender (P < 0.001), age at diagnosis (P < 0.001), smoking history (P < 0.001), drinking history (P < 0.001), tumor length (P = 0.013), tumor location (P = 0.037) and treatment type (P = 0.001). The association between MVV and overall postoperative complications (P = 0.243, Table 2) were not observed, and MVV was not associated with postoperative pulmonary complications (P = 0.843). The HALP score was notably associated with gender (P < 0.001), age at diagnosis (P = 0.035), tumor length (P < 0.001) and invasion depth (P = 0.001). The association of patients’ clinical features with MVV and HALP score was shown in Table 2.
Figure 1.
Receiver operating characteristic curves for 5-year overall survival in esophageal squamous cell carcinoma patients. A: Receiver operating characteristic (ROC) curve of maximal voluntary ventilation (MVV) for 5-year overall survival; B: ROC curve of hemoglobin, albumin, lymphocytes and platelets (HALP) score for 5-year overall survival. AUC: Area under the curve; CI: Confidence interval.
Table 2.
Association of maximal voluntary ventilation and hemoglobin, albumin, lymphocytes and platelets score with basic clinical factors, n (%)
| Variable |
MVV
|
HALP score
|
|||||
|
|
≥ 85.2
|
< 85.2
|
P
value
|
≥ 38.8
|
< 38.8
|
P
value
|
|
| Gender | < 0.001 | < 0.001 | |||||
| Male | 404 (97.1) | 105 (25.1) | 319 (66.2) | 190 (54.0) | |||
| Female | 12 (2.9) | 313 (74.9) | 163 (33.8) | 162 (46.0) | |||
| Age at diagnosis in yr | < 0.001 | 0.035 | |||||
| < 60 | 250 (60.1) | 149 (35.6) | 246 (51.0) | 153 (43.5) | |||
| ≥ 60 | 166 (39.9) | 269 (64.4) | 236 (49.0) | 199 (56.5) | |||
| Smoking history | < 0.001 | 0.107 | |||||
| No | 108 (26.0) | 326 (78.0) | 239 (49.6) | 195 (55.4) | |||
| Yes | 308 (74.0) | 92 (22.0) | 243 (50.4) | 157 (44.6) | |||
| Drinking history | < 0.001 | 0.082 | |||||
| No | 209 (50.2) | 365 (87.3) | 320 (66.4) | 254 (72.2) | |||
| Yes | 207 (49.8) | 53 (12.7) | 162 (33.6) | 98 (27.8) | |||
| Tumor length in cm | 0.013 | < 0.001 | |||||
| < 4 | 175 (42.1) | 212 (50.7) | 249 (51.7) | 138 (39.2) | |||
| ≥ 4 | 241 (57.9) | 206 (49.3) | 233(48.3) | 214 (60.8) | |||
| Tumor location | 0.037 | 0.733 | |||||
| Upper | 75 (18.0) | 65 (15.5) | 85 (17.6) | 55 (15.6) | |||
| Middle | 257 (61.8) | 292(69.9) | 315 (65.4) | 234 (66.5) | |||
| Lower | 84 (20.2) | 61 (14.6) | 82 (17.0) | 63 (17.9) | |||
| Differentiation | 0.490 | 0.183 | |||||
| Well | 15 (3.6) | 22 (5.3) | 16 (3.3) | 21 (6.0) | |||
| Moderate | 229 (55.1) | 230 (55.0) | 267 (55.4) | 192 (54.5) | |||
| Poor | 172 (41.3) | 166 (39.7) | 199 (41.3) | 139 (39.5) | |||
| Invasion depth | 0.366 | 0.001 | |||||
| pT1 | 47 (11.3) | 61 (14.6) | 69 (14.3) | 39 (11.1) | |||
| pT2 | 122 (29.3) | 118 (28.2) | 158 (32.8) | 82 (23.3) | |||
| pT3 | 247 (59.4) | 239 (57.2) | 255 (52.9) | 231 (65.6) | |||
| Lymph node metastasis | 0.163 | 0.672 | |||||
| pN0 | 242 (58.2) | 252 (60.3) | 290 (60.2) | 204 (57.9) | |||
| pN1 | 107 (25.7) | 83 (19.8) | 112 (23.2) | 78 (22.2) | |||
| pN2 | 53 (12.7) | 68 (16.3) | 64 (13.3) | 57 (16.2) | |||
| pN3 | 14 (3.4) | 15 (3.6) | 16 (3.3) | 13 (3.7) | |||
| TNM stage | 0.541 | 0.084 | |||||
| I | 45 (10.8) | 55 (13.2) | 62 (12.9) | 38 (10.8) | |||
| II | 232 (55.8) | 222 (53.1) | 273 (56.6) | 181 (51.4) | |||
| III | 139 (33.4) | 141 (33.7) | 147 (30.5) | 133 (37.8) | |||
| Surgical approach | 0.478 | 0.718 | |||||
| Left | 374 (89.9) | 382 (91.4) | 435 (90.2) | 321 (91.2) | |||
| Right | 42 (10.1) | 36 (8.6) | 47 (9.8) | 31 (8.8) | |||
| Treatment type | 0.001 | 0.353 | |||||
| Surgery | 305 (73.3) | 345 (82.5) | 370 (76.8) | 280 (79.5) | |||
| Surgery + adjuvant RT/CT | 111 (26.7) | 73 (17.5) | 112 (23.2) | 72 (20.5) | |||
| Overall complications | 0.243 | 0.525 | |||||
| No | 334 (80.3) | 349 (83.5) | 391 (81.1) | 292 (83.0) | |||
| Yes | 82 (19.7) | 69 (16.5) | 91 (18.9) | 60 (17.0) | |||
CT: Chemotherapy; HALP: Hemoglobin, albumin, lymphocytes and platelets; MVV: Maximal voluntary ventilation; RT: Radiotherapy; TNM: Tumor-node-metastasis.
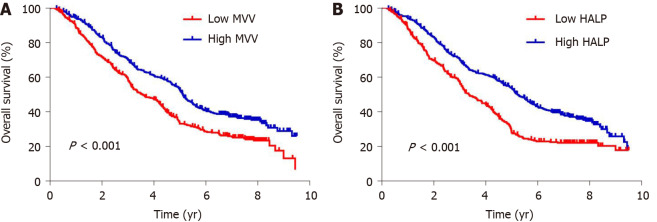
Association of MVV and HALP score with overall survival
To evaluate the potential association of clinical factors with overall survival, we performed univariate Cox regression analysis and observed a significant association with age at diagnosis (P < 0.001), tumor length (P < 0.001), differentiation (P < 0.001), invasion depth (P < 0.001), lymph node metastasis (P < 0.001) and postoperative complications (P < 0.001) (Table 3). We also observed an association between survival and MVV (P < 0.001) and HALP score (P < 0.001) (Table 3). Kaplan-Meier analysis using the log-rank test showed that low MVV (P < 0.001, Figure 2) and low HALP score (P < 0.001, Figure 2) predicted a worse overall survival. By adjusting the clinical factors from the multivariate Cox regression analysis, the association of low MVV and low HALP score with poor survival remained significantly with P < 0.001 (hazard ratio: 1.451, 95% confidence interval: 1.208-1.743) and P < 0.001 (hazard ratio: 1.537, 95% confidence interval: 1.287-1.836) (Table 3), respectively, suggesting that low MVV and HALP score were independent risk factors for overall survival. Gender, smoking history, drinking history, tumor location, differentiation, surgical approach and treatment type were not independently associated with overall survival (all P > 0.05), whereas age at diagnosis, tumor length, invasion depth, lymph node metastasis, MVV, HALP score and postoperative complications were independent predictive factors for overall survival (all P < 0.05) (Table 3).
Table 3.
Univariate and multivariate analyses for patients' overall survival with esophageal squamous cell carcinoma
| Variable |
Univariate analysis
|
Multivariate analysis
|
||
|
|
HR (95%CI)
|
P
value
|
HR (95%CI)
|
P
value
|
| Gender | 0.924 | |||
| Female | 1 | |||
| Male | 0.991 (0.831-1.183) | |||
| Age at diagnosis in yr | < 0.001 | < 0.001 | ||
| < 60 | 1 | 1 | ||
| ≥ 60 | 1.613 (1.355-1.920) | 1.388 (1.155-1.668) | ||
| Smoking history | 0.760 | |||
| No | 1 | |||
| Yes | 0.974 (0.821-1.155) | |||
| Drinking history | 0.293 | |||
| No | 1 | |||
| Yes | 0.905 (0.752-1.090) | |||
| Tumor length in cm | < 0.001 | 0.018 | ||
| < 4 | 1 | 1 | ||
| ≥ 4 | 1.691 (1.419-2.016) | 1.259 (1.040-1.525) | ||
| Tumor location | 0.064 | |||
| Upper | 1 | |||
| Middle | 0.782 (0.623-0.982) | |||
| Lower | 0.733 (0.549-0.979) | |||
| Differentiation | < 0.001 | 0.308 | ||
| Well | 1 | 1 | ||
| Moderate | 1.308 (0.839-2.039) | 1.189 (0.759-1.865) | ||
| Poor | 1.829 (1.169-2.862) | 1.334 (0.841-2.118) | ||
| Invasion depth | < 0.001 | 0.001 | ||
| pT1 | 1 | 1 | ||
| pT2 | 2.100 (1.491-2.958) | 1.671 (1.173-2.379) | ||
| pT3 | 2.769 (2.007-3.819) | 1.926 (1.371-2.706) | ||
| Lymph node metastasis | < 0.001 | < 0.001 | ||
| pN0 | 1 | 1 | ||
| pN1 | 1.803 (1.463-2.222) | 1.695 (1.362-2.109) | ||
| pN2 | 2.809 (2.224-3.549) | 2.281 (1.780-2.922) | ||
| pN3 | 3.857 (2.571-5.787) | 2.945 (1.926-4.504) | ||
| Surgical approach | 0.130 | |||
| Left | 1 | |||
| Right | 1.250 (0.936-1.669) | |||
| Treatment type | 0.512 | |||
| Surgery | 1 | |||
| Surgery + adjuvant RT/CT | 1.072 (0.871-1.319) | |||
| MVV | < 0.001 | < 0.001 | ||
| ≥ 85.2 | 1 | 1 | ||
| < 85.2 | 1.487 (1.252-1.767) | 1.451 (1.208-1.743) | ||
| HALP score | < 0.001 | < 0.001 | ||
| ≥ 38.8 | 1 | 1 | ||
| < 38.8 | 1.617 (1.361-1.922) | 1.537 (1.287-1.836) | ||
| Overall complications | < 0.001 | < 0.001 | ||
| No | 1 | 1 | ||
| Yes | 1.456 (1.179-1.799) | 1.515 (1.218-1.884) | ||
CI: Confidence interval; CT: Chemotherapy; HALP: Hemoglobin, albumin, lymphocytes and platelets; HR: Hazard ratio; MVV: Maximal voluntary ventilation; RT: Radiotherapy.
Figure 2.
Kaplan-Meier curves for overall survival in esophageal squamous cell carcinoma patients. A: Kaplan-Meier curves of overall survival according to maximal voluntary ventilation (MVV) in esophageal squamous cell carcinoma (ESCC) patients; B: Kaplan-Meier curves of overall survival according to the hemoglobin, albumin, lymphocytes and platelets (HALP) score in ESCC patients.
Predictive value of the combination of MVV with HALP score for overall survival
Since both MVV and HALP score were independent predictive factors, we next explored the cumulative effect of MVV and HALP score (coMVV-HALP) on overall survival. We defined the following: (1) The coMVV-HALP score was 0 when patients had both low MVV and HALP score; (2) The coMVV-HALP score was 1 when patients had either low MVV or HALP score; and (3) The coMVV-HALP score was 2 when patients had both high MVV and HALP score. Then, we performed ROC analysis to evaluate the predictive value of the coMVV-HALP score for 5-year overall survival. We found that the AUC for the coMVV-HALP score was 0.681 (P < 0.001, Figure 3), which indicated better prognostic value than TNM (AUC: 0.659) and HALP score (AUC: 0.646) or MVV (AUC: 0.631) alone.
Figure 3.
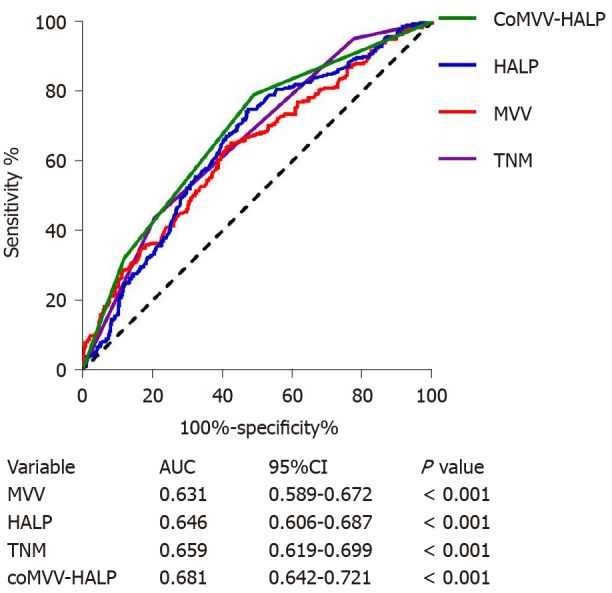
Receiver operating characteristic curves of maximal voluntary ventilation, hemoglobin, albumin, lymphocytes and platelets score, tumor-node-metastasis and maximal voluntary ventilation and hemoglobin, albumin, lymphocytes and platelets score for predicting 5-year overall survival in esophageal squamous cell carcinoma patients. MVV: Maximal voluntary ventilation; HALP: Hemoglobin, albumin, lymphocytes and platelets; CoMVV-HALP: MVV and HALP; AUC: Area under the curve; CI: Confidence interval; TNM: Tumor-node-metastasis.
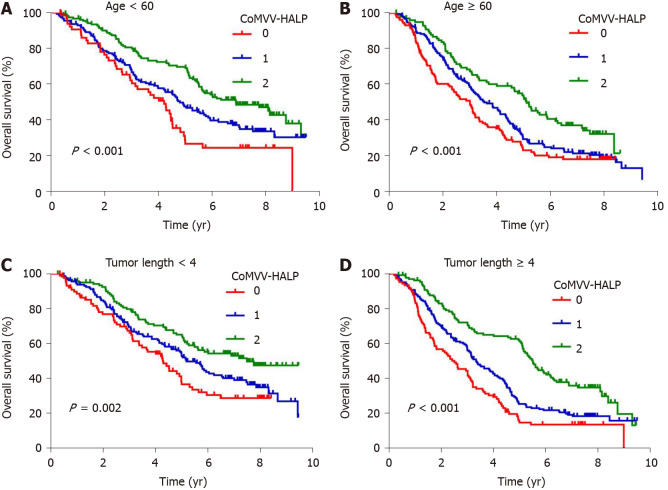
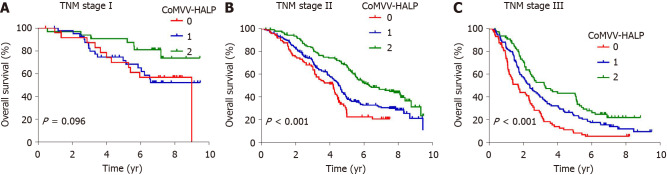
Kaplan-Meier analysis using the log-rank test showed that a low coMVV-HALP score predicted poor overall survival (P < 0.001, Figure 4) in ESCC patients. As age at diagnosis, tumor length, invasion depth and lymph node metastasis were independent predictive factors for overall survival, we then performed further stratification analysis for the ESCC patients according to age, tumor length and TNM to explore the predictive value of the coMVV-HALP score in different groups. Stratification analysis by age at diagnosis and tumor length accordingly showed that patients with coMVV-HALP score = 0 had a worse overall survival than patients with coMVV-HALP score = 1 or 2 in groups with age < 60 (n = 379, P < 0.001, Figure 5), age ≥ 60 (n = 420, P < 0.001, Figure 5), tumor length < 4 cm (n = 374, P = 0.002, Figure 5) and tumor length ≥ 4 cm (n = 425, P < 0.001, Figure 5). Stratification analysis by TNM stage showed no significant difference in overall survival among patients with different coMVV-HALP scores (0, 1, 2) in stage I (n = 98, P = 0.096, Figure 6). However, we found that, among patients in stage II (n = 437, P < 0.001, Figure 6) and III (n = 264, P < 0.001, Figure 6), those with coMVV-HALP score = 0 had a worse overall survival than those with coMVV-HALP score = 1 or 2. Multivariate analysis further showed that a low coMVV-HALP score was an independent risk factor for poor overall survival (P < 0.001, Supplementary Table 1).
Figure 4.
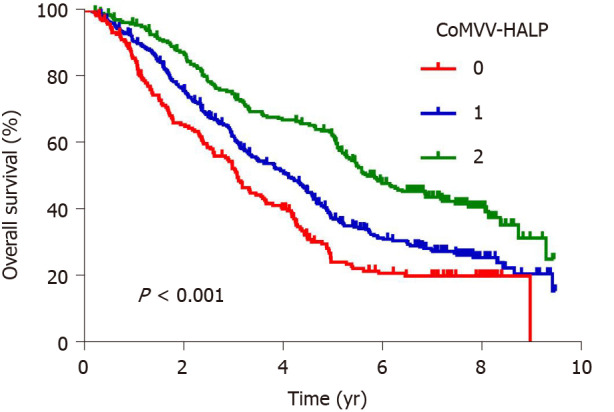
Kaplan-Meier curves of overall survival according to the maximal voluntary ventilation and hemoglobin, albumin, lymphocytes and platelets score in esophageal squamous cell carcinoma patients. MVV: Maximal voluntary ventilation; HALP: Hemoglobin, albumin, lymphocytes and platelets; coMVV-HALP: MVV and HALP.
Figure 5.
Kaplan-Meier curves of overall survival according to the maximal voluntary ventilation and hemoglobin, albumin, lymphocytes and platelets score in subgroups of esophageal squamous cell carcinoma patients with different ages at diagnosis and tumor lengths. A: Kaplan-Meier curves of overall survival according to maximal voluntary ventilation (MVV) and hemoglobin, albumin, lymphocytes and platelets (coMVV-HALP) score in group with age < 60; B: Kaplan-Meier curves of overall survival according to coMVV-HALP score in the group with age ≥ 60; C: Kaplan-Meier curves of overall survival according to coMVV-HALP score in the group with tumor length < 4 cm; D: Kaplan-Meier curves of overall survival according to coMVV-HALP score in the group with tumor length ≥ 4 cm.
Figure 6.
Kaplan-Meier curves of overall survival according to the maximal voluntary ventilation and hemoglobin, albumin, lymphocytes and platelets score in subgroups of esophageal squamous cell carcinoma patients with different tumor-node-metastasis stages. A: Kaplan-Meier curves of overall survival according to maximal voluntary ventilation (MVV) and hemoglobin, albumin, lymphocytes and platelets (coMVV-HALP) score in patients with tumor-node-metastasis (TNM) stage I; B: Kaplan-Meier curves of overall survival according to coMVV-HALP score in TNM stage II; C: Kaplan-Meier curves of overall survival according to coMVV-HALP score in TNM stage III.
DISCUSSION
Reliable clinical factors that predict patients’ overall survival are important for clinicians to make informed treatment decisions. In this study, we investigated the prognostic value of preoperative pulmonary evaluation with MVV and HALP score for predicting the long-term survival of ESCC patients. Our data showed that MVV, HALP score and their combination were independent prognostic factors. If further validated, this score may be used clinically to overcome some limitations of the TNM staging system in assessing ESCC prognosis.
Numerous studies have focused mainly on the prognostic value of preoperative pulmonary function in lung cancer but rarely in digestive cancers. These studies have clearly shown that preoperative pulmonary evaluation could be used as a predictor for postoperative complications and survival in lung cancer[9,23]. As described previously, FVC and VC have been demonstrated as predictors of postoperative complications and the survival in gastric cancer[10] and Japanese esophageal cancer[11], respectively. In the present study, we did not find the association between MVV and postoperative complications. One possibility was that we had a different source of patients, inclusion and exclusion criteria as well as sample size. In this study, we found that low MVV was an independent risk factor for the overall survival of Chinese ESCC patients. Although the mechanism of this association between lung function and cancer prognosis is unclear, multiple studies have demonstrated that impaired lung function is associated with mortality risk in the general population[7,8]. Additionally, Sarkar et al[24] elaborated a mechanism in which abnormal function of the respiratory system could lead to the development of hypoxemia and its detrimental consequences. Muz et al[25] have reported that tumor-hypoxia plays a vital role in cell mobility and metastasis in cancer progression. It was also shown that hypoxia was correlated with poor prognosis in cancer patients[26]. Moreover, pulmonary rehabilitation is important for lung cancer patients to improve pulmonary capacity and quality of life[27]. A study has suggested that pulmonary rehabilitation benefits cancer patients and should be applied to the entire process of cancer care[28]. Therefore, MVV may influence the capacity of oxygen supply for the body and then further affect the prognosis.
The HALP score is a new combined index and its prognostic value has been investigated in many cancers, but its role in ESCC patients who underwent esophagectomy has not been studied. Our study showed that the HALP score was an independent factor for overall survival in this group of ESCC patients. The result is further supported by a study that investigated the role of the HALP score in 39 male ESCC patients receiving platinum-based chemotherapy[29]. The survival association may be explained by the following findings: (1) Studies have shown that anemia affects patients’ disease progression, treatment, quality of life and survival[13,30], and anemia before radiotherapy predicts poor survival for ESCC patients[31]; (2) Low serum albumin levels are common in many advanced cancers, and some studies suggest that albumin may be a source of energy for cancer growth[32] and metabolism[33]. Importantly, it has been shown that low albumin is a risk factor for the survival outcome in ESCC[14]; (3) A systemic review has demonstrated that lymphocytes play an important role in mediating tumor suppression and controlling tumor growth[34]. A low preoperative lymphocyte count could serve as a risk factor for a variety of cancers including ESCC[35,36]; and (4) Platelets may protect tumor cells from immunosurveillance and mediate cancer metastasis[37]. Clearly, these accumulated studies have provided strong evidence showing the prognostic value of the HALP score, which partly reflects the status of inflammation and nutrition.
By combining the MVV and HALP score, we developed a predictive coMVV-HALP score for ESCC survival, and it has a better predictive value than TNM. To the best of our knowledge, this study is the first to investigate the value of MVV and HALP score combination, which reflects the status of inflammation, nutrition and pulmonary function of ESCC patients. It is well known that esophageal cancer is a systemic disease that impacts many systems in the human body. CoMVV-HALP score is based on the routine data from the preoperative blood test and pulmonary evaluation. TNM staging system is well known as the criteria to assess the prognosis of the esophageal cancer patients, but it relies on the complete postoperative pathological information. CoMVV-HALP score may provide a new method to evaluate the prognosis of the patients before surgery. Therefore, the multivariate model integrating MVV with the HALP score may partly compensate for the limitation of the TNM staging system. This score can be easily calculated since individual clinical factors are conveniently available using a less invasive approach. These clinical factors can also be monitored cost-effectively during the course of disease progression.
This study also has some limitations: (1) Since it is a retrospective study from a single center involving different pathologists, physicians and technicians, there might be some bias of the uniformity in determining some clinical factors; and (2) The sample size was relatively small and included only ESCC with no other types of esophageal cancer. Further prospective studies using a large sample size from more centers are needed to confirm this new finding.
CONCLUSION
MVV and HALP score, alone or in combination are simple and promising clinical predictors. Once validated in large cohorts, these clinical factors should be taken into account when assessing the prognosis of ESCC patients.
ARTICLE HIGHLIGHTS
Research background
Preoperative pulmonary function plays an important role in selecting surgical candidates and assessing postoperative complications. Nutritional and systemic inflammation parameters are vital to cancer survival, and the combination of these parameters improves the prognostic value. The hemoglobin, albumin, lymphocytes and platelets (HALP) score is a novel prognostic indicator to reflect the nutritional and inflammation status.
Research motivation
The prognostic values of preoperative pulmonary function and the HALP score for survival in esophageal squamous cell carcinoma (ESCC) are unclear.
Research objectives
This study aimed to investigate the predictive values of preoperative pulmonary function and the HALP score for survival in ESCC patients.
Research methods
The predictive values of preoperative pulmonary function and the HALP score for long-term overall survival were performed in 834 ESCC patients who underwent radical esophagectomy.
Research results
Low maximal voluntary ventilation (MVV) and the HALP score were both independent risk factors for overall survival (all P < 0.001). The combination of MVV and HALP score (coMVV-HALP) improved the prediction performance for overall survival compared to tumor-node-metastasis, and low coMVV-HALP score was an independent risk factor for poor overall survival (P < 0.001).
Research conclusions
MVV, HALP score and their combination are simple and promising clinical markers to predict overall survival of ESCC patients.
Research perspectives
MVV and HALP score, alone or in combination, are simple and promising clinical predictors. Once validated in large cohorts, these clinical factors should be taken into account when assessing the prognosis of ESCC patients.
ACKNOWLEDGEMENTS
We thank Dr. Tian YJ (H. Lee Moffitt Cancer Center, United States) for English editing and thoughtful comments for this manuscript; and Professor Shi XZ (Department of Epidemiology and Biostatistics, College of Public Health in Zhengzhou University) for help in statistical analysis.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Zhengzhou University.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: We have no potential conflicts of interest to disclose.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: The Chinese Society for Diseases of the Esophagus; American Association for Cancer Research; and International Society for Diseases of the Esophagus.
Peer-review started: October 13, 2020
First decision: December 3, 2020
Article in press: December 24, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cho YS, De Nardi P S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
Contributor Information
Shou-Jia Hu, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Xue-Ke Zhao, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Xin Song, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Ling-Ling Lei, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Wen-Li Han, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Rui-Hua Xu, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Ran Wang, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Fu-You Zhou, Department of Thoracic Surgery, Anyang Tumor Hospital, Anyang 455000, Henan Province, China.
Liang Wang, Department of Tumor Biology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL 33612, United States.
Li-Dong Wang, State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University, Zhengzhou 450052, Henan Province, China. ldwang2007@126.com.
References
- 1.Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–2396. doi: 10.1016/S0140-6736(17)31462-9. [DOI] [PubMed] [Google Scholar]
- 2.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 3.Smetana GW. Preoperative pulmonary evaluation. N Engl J Med. 1999;340:937–944. doi: 10.1056/NEJM199903253401207. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg. 2011;91:1494–1500; discussion 1500. doi: 10.1016/j.athoracsur.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Reinersman JM, Allen MS, Deschamps C, Ferguson MK, Nichols FC, Shen KR, Wigle DA, Cassivi SD. External validation of the Ferguson pulmonary risk score for predicting major pulmonary complications after oesophagectomy†. Eur J Cardiothorac Surg. 2016;49:333–338. doi: 10.1093/ejcts/ezv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goense L, Meziani J, Bülbül M, Braithwaite SA, van Hillegersberg R, Ruurda JP. Pulmonary diffusion capacity predicts major complications after esophagectomy for patients with esophageal cancer. Dis Esophagus. 2019;32 doi: 10.1093/dote/doy082. [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58:388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey MS, Jankowich MD. The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest. 2016;149:238–251. doi: 10.1378/chest.15-1045. [DOI] [PubMed] [Google Scholar]
- 9.Lee SY, Choi YJ, Seo JH, Lee SY, Kim JS, Kang EJ. Pulmonary function is implicated in the prognosis of metastatic non-small cell lung cancer but not in extended disease small cell lung cancer. J Thorac Dis. 2019;11:4562–4572. doi: 10.21037/jtd.2019.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng F, Tian Y, Zang Y, Sun L, Hong L, Yang J, Guo M, Lian X, Fan D, Zhang H. Low forced vital capacity predicts poor prognosis in gastric cancer patients. Oncotarget. 2017;8:28897–28905. doi: 10.18632/oncotarget.15953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugawara K, Mori K, Okumura Y, Yagi K, Aikou S, Uemura Y, Yamashita H, Seto Y. Preoperative Low Vital Capacity Influences Survival After Esophagectomy for Patients with Esophageal Carcinoma. World J Surg. 2020;44:2305–2313. doi: 10.1007/s00268-020-05450-0. [DOI] [PubMed] [Google Scholar]
- 12.Suh MR, Kim DH, Jung J, Kim B, Lee JW, Choi WA, Kang SW. Clinical implication of maximal voluntary ventilation in myotonic muscular dystrophy. Medicine (Baltimore) 2019;98:e15321. doi: 10.1097/MD.0000000000015321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang XZ, Yang YC, Chen Y, Wu CC, Lin RF, Wang ZN, Zhang X. Preoperative Anemia or Low Hemoglobin Predicts Poor Prognosis in Gastric Cancer Patients: A Meta-Analysis. Dis Markers. 2019;2019:7606128. doi: 10.1155/2019/7606128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu N, Chen G, Hu H, Pang L, Chen Z. Low pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomas. Nutr Cancer. 2015;67:481–485. doi: 10.1080/01635581.2015.1004726. [DOI] [PubMed] [Google Scholar]
- 15.Rho SY, Hwang HK, Chong JU, Yoon DS, Lee WJ, Kang CM. Association of preoperative total lymphocyte count with prognosis in resected left-sided pancreatic cancer. ANZ J Surg. 2019;89:503–508. doi: 10.1111/ans.15030. [DOI] [PubMed] [Google Scholar]
- 16.Rachidi S, Li H, Wallace K, Li Z, Balch C, Lautenschlaeger T. Preoperative platelet counts and postoperative outcomes in cancer surgery: a multicenter, retrospective cohort study. Platelets. 2020;31:79–87. doi: 10.1080/09537104.2019.1573977. [DOI] [PubMed] [Google Scholar]
- 17.Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, Fu JH, Tan ZH, Meng YQ, Fu XY, Ma QL, Lin P. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:5591–5597. doi: 10.3748/wjg.v21.i18.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, Uchida E. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2016;23:646–654. doi: 10.1245/s10434-015-4869-5. [DOI] [PubMed] [Google Scholar]
- 19.Xue Y, Zhou X, Xue L, Zhou R, Luo J. The role of pretreatment prognostic nutritional index in esophageal cancer: A meta-analysis. J Cell Physiol. 2019;234:19655–19662. doi: 10.1002/jcp.28565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, Gao HL, Jiang W, Zhang WH, Li TJ, Ni QX, Liu L, Yu XJ. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. 2020;26:828–838. doi: 10.3748/wjg.v26.i8.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng D, Zhang CJ, Gong YQ, Hao H, Guan B, Li XS, Zhou LQ. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. 2018;8:794. doi: 10.1038/s41598-018-19146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, Zhang Z, Jin L, Yang B, Ye L, Yao X. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score is a Novel Significant Prognostic Factor for Patients with Metastatic Prostate Cancer Undergoing Cytoreductive Radical Prostatectomy. J Cancer. 2019;10:81–91. doi: 10.7150/jca.27210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho O, Oh YT, Chun M, Noh OK, Heo JS. Prognostic implication of FEV1/FVC ratio for limited-stage small cell lung cancer. J Thorac Dis. 2018;10:1797–1805. doi: 10.21037/jtd.2018.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkar M, Niranjan N, Banyal PK. Mechanisms of hypoxemia. Lung India. 2017;34:47–60. doi: 10.4103/0970-2113.197116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Liu X, Rice SJ, Belani CP. Pulmonary Rehabilitation in Lung Cancer. PM R. 2016;8:990–996. doi: 10.1016/j.pmrj.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Morris GS, Gallagher GH, Baxter MF, Brueilly KE, Scheetz JS, Ahmed MM, Shannon VR. Pulmonary rehabilitation improves functional status in oncology patients. Arch Phys Med Rehabil. 2009;90:837–841. doi: 10.1016/j.apmr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2017;46:75–79. doi: 10.1016/j.intimp.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Madeddu C, Gramignano G, Astara G, Demontis R, Sanna E, Atzeni V, Macciò A. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front Physiol. 2018;9:1294. doi: 10.3389/fphys.2018.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Cheng F, Cao L, Wang S, Zhou W, Ma W. A retrospective study: the prevalence and prognostic value of anemia in patients undergoing radiotherapy for esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:244. doi: 10.1186/1477-7819-12-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stehle G, Sinn H, Wunder A, Schrenk HH, Stewart JC, Hartung G, Maier-Borst W, Heene DL. Plasma protein (albumin) catabolism by the tumor itself--implications for tumor metabolism and the genesis of cachexia. Crit Rev Oncol Hematol. 1997;26:77–100. doi: 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 33.Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:299. doi: 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018;75:689–713. doi: 10.1007/s00018-017-2686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong J, Chen X, Gao W, Zhu S, Wu J, Huang O, He J, Zhu L, Chen W, Li Y, Fei X, Lin L, Shen K. A high absolute lymphocyte count predicts a poor prognosis in HER-2- positive breast cancer patients treated with trastuzumab. Cancer Manag Res. 2019;11:3371–3379. doi: 10.2147/CMAR.S187233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2014;93:e257. doi: 10.1097/MD.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11:125. doi: 10.1186/s13045-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]