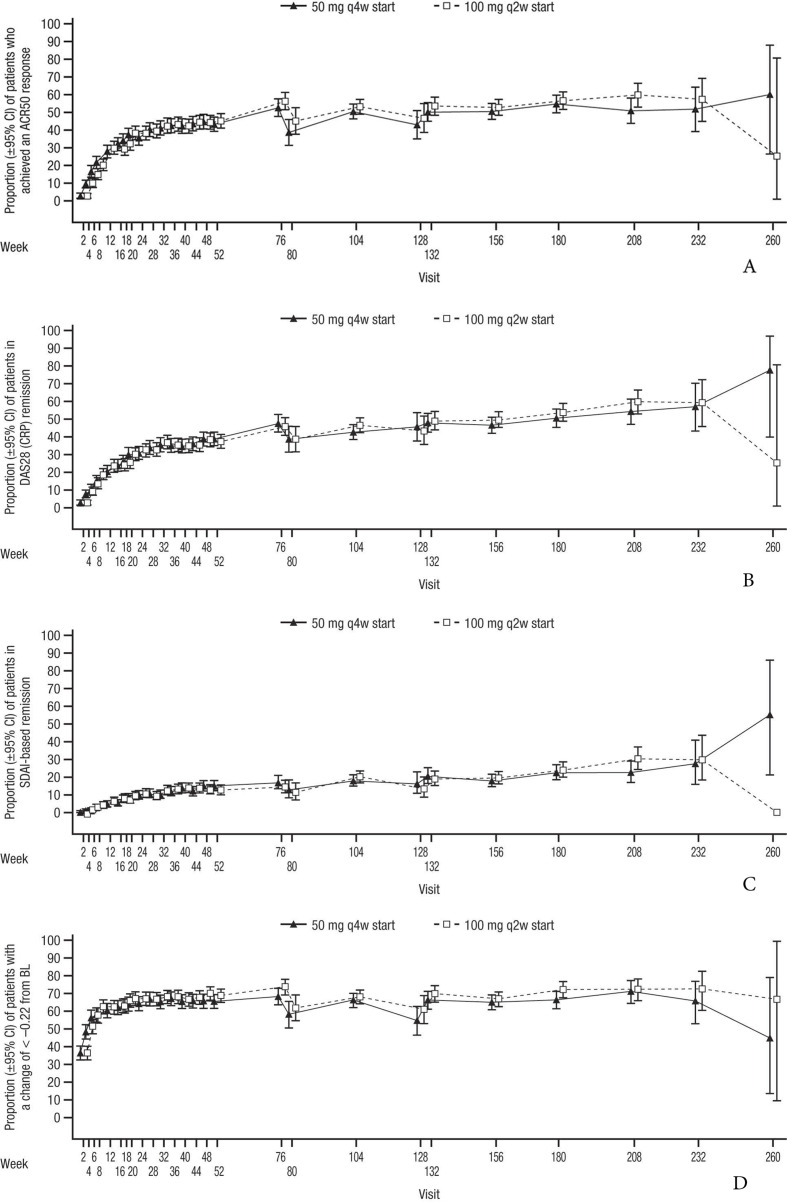
Figure 2.
(A) Proportion of patients with ACR50 response from baseline of primary studies to the end of the SIRROUND-LTE study period. (B) Proportion of patients with DAS28 (CRP) remission from baseline of primary studies to the end of the SIRROUND-LTE study period. (C) Proportion of patients with SDAI-based ACR/EULAR remission from baseline of primary studies to the end of the SIRROUND-LTE study period. (D) Proportion of patients with HAQ-DI response from baseline of primary studies to the end of the SIRROUND-LTE study period. ACR, American College of Rheumatology; BL, baseline; DAS28 (CRP), Disease Activity Index Score 28 (C reactive protein); HAQ-DI, Health Assessment Questionnaire-Disability Index; LTE, long-term extension; q2w, every 2 weeks; q4w, every 4 weeks; SDAI, Simplified Disease Activity Index.