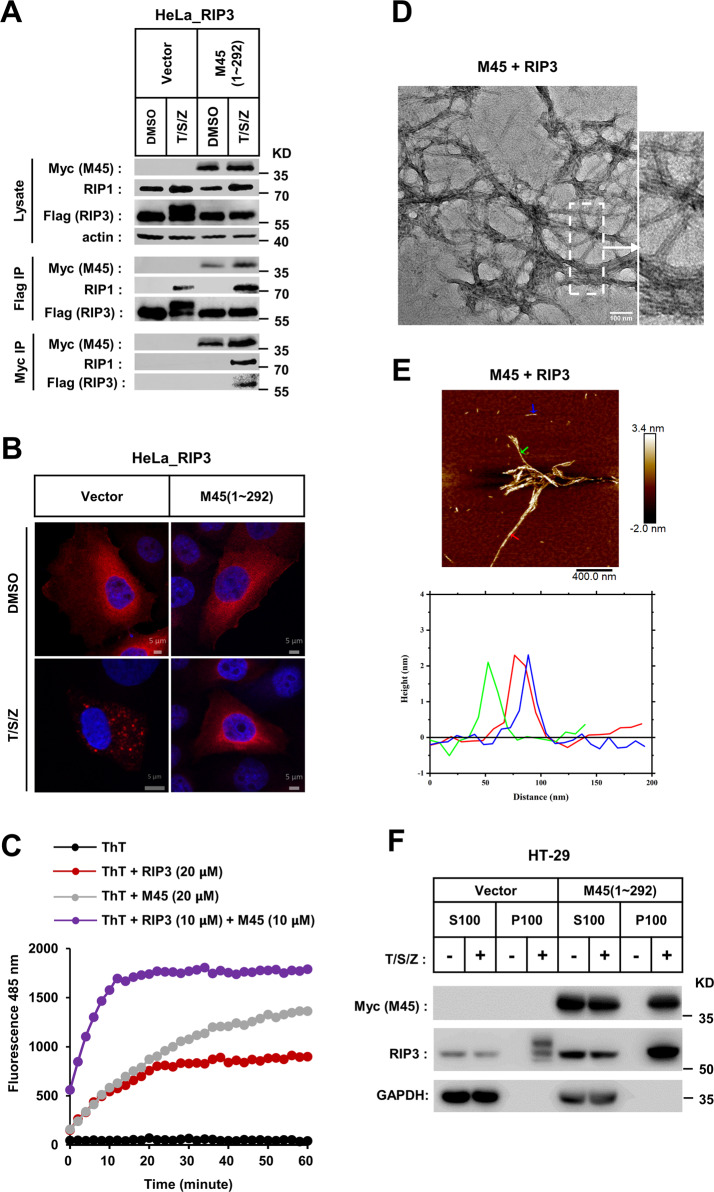
Fig. 5. M45–RHIM prevents RIP3 fibrils from self-assembling into cellular puncta.
a M45 do not repress RIP1-RIP3 binding in necrosome. RIP3-HeLa cells were infected with empty virus (vector) or lentiviral virus encoding Myc-tagged M45 (1–292) in the presence of T/S/Z for 6 h. Whole-cell lysates were subjected to immunoprecipitation with anti-Flag M2 beads or anti-Myc beads. The total cell lysates and immunoprecipitates were immunoblotted with the indicated antibodies. b M45 can block TNF-induced cellular RIP3 puncta formation. RIP3-HeLa cells infected with empty virus (vector) or lentiviral virus encoding Myc-tagged M45 (1–292) were treated as the indicated for 6 h. The distribution of RIP3 (red) was detected by immunofluorescence as described in “Materials and Methods.” The scale bar represents 5 μm. c Assembly profile of different peptides (M45 + RIP3) (purple); M45 (gray); RIP3 (red) monitored by ThT fluorescence binding assay as described in “Materials and Methods.” d EM image of M45 + RIP3 heteromeric amyloid fibrils formed by dilution equimolar mixed RIP3 and M45 stock solution into water as described in “Materials and Methods” (scale bar 100 nm). e AFM images and the height profile of RIP3–M45 amyloid fibrils. The arrow indicated the position to obtain the height profile data. Hetero RIP3–M45 fibril with a uniform height of 2.1 ± 0.3 nm. f Analysis of intracellular RIP3 aggregation. HT-29 cells infected with the indicated lentivirus were treated with T/S/Z for 8 h. The cells were harvested and homogenized, then separated into the indicated fractions as described in “Materials and Methods.” The distribution of RIP3 and M45 in indicated fractions was analyzed by western blot. S supernatant, P precipitate.