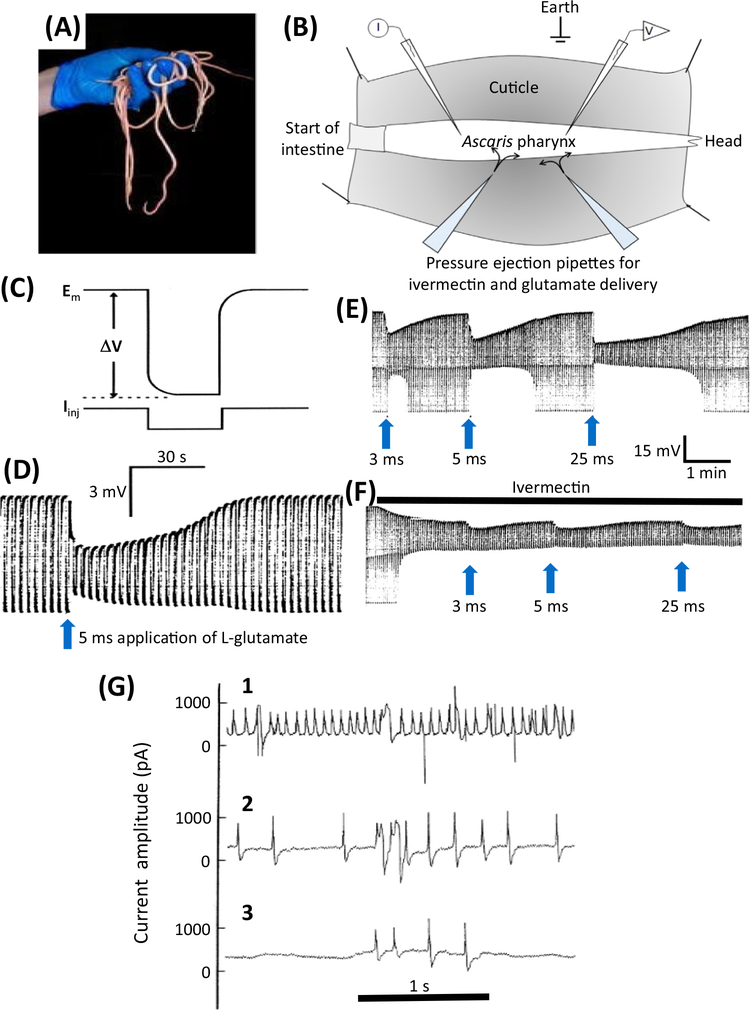
Figure 2. Electrophysiologic Effects of Ivermectin in Parasitic Nematodes.
(A) Ascaris suum is a large nematode that is tractable for electrophysiologic recordings. (B) A diagram of the dissected A. suum pharynx. Two glass micropipettes are placed in the pharynx, one for injecting rectangular current pulses (I) and one for recording the membrane potential (V). Two ‘puffing’ microelectrodes are used to apply, separately, glutamate or ivermectin at different time intervals. (C) Higher time resolution recording showing the injection of a hyperpolarizing rectangular current pulse (Iinj) and the membrane potential (Em) and response to the injected current (ΔV) allowing the input conductance (= 1/resistance) of the pharynx to be measured. (D) A 5 ms puff of 0.5 M glutamate produces a hyperpolarization due to the entry of Cl− (upper envelope of the trace) and a reduction in the width of the trace as the size of the membrane potential change (ΔV) is reduced as the glutamate-gated chloride (GluCl) channels open and increase the conductance of the pharyngeal muscle membrane. (E) Effects of glutamate. The trace shows the membrane potential and input conductance responses to 3 ms, 5 ms, and 25 ms ‘puffs’ of 0.5 M glutamate from a micropipette, which produces maximal changes in input conductance of 16 μS, 33 μS, and 60 μS, respectively. Longer applications of glutamate did not produce an increase in response in this experiment, showing that the 25 μs response led to a maximum response. F) The effect of a continuous ‘puff’ of 100 μM ivermectin applied also by pressure ejection from a micropipette. Once the conductance change produced by the ivermectin reached a stable position of 32.7 μS, glutamate was again applied in the same controlled way using the same application times; this produced a maximum conductance change of 28 μS with a 25 ms puff application. The maximum conductance change obtained with the coapplication of glutamate and ivermectin was 60 μS, virtually the same conductance (cf. 60 μS) change produced after a high-dose application of glutamate. The fact that coapplication did not produce an effect greater than the high dose of glutamate was interpreted as indicating that these two substances activate the same ion channel – because application of two separate ion channels by glutamate and ivermectin at high concentrations would produce an additive response. (G) Electropharyngeograms from Trichostrongylus colubriformis A before, 5 min after, and 15 min after adding 100 nM ivermectin to the bath (from [106]). A patch-pipette was used to record the currents elicited by the pumping pharynx by sucking the head of the worm into the patch-pipette. (1) Control recording before application of 100 nM ivermectin; (2) 5 min after application of 100 nM ivermectin; (3) 15 min after application of 100 nM ivermectin.