Abstract
Background
Bovine viral diarrhea virus (BVDV) is an important global viral pathogen of cattle and other ruminants. To survey the infection rate and genetic diversity of BVDV in western China, a total of 1234 serum samples from 17 herds of dairy cattle, beef cattle and yak in 4 provinces were collected in 2019.
Results
All the 1234 serum samples were screened individually for BVDV by RT-PCR. Our results demonstrated that the average positive rate of BVDV was 7.2% (89/1234) in animals and 82.4% (14/17) in herds. Thirteen BVDV strains were isolated from RT-PCR positive clinical samples and they were all NCP biotype. BVDV-1a and 1c subgenotypes were identified from 22 selected virus isolates in 14 BVDV-positive herds. These results confirmed that BVDV-1a and BVDV-1c were circulating in western China, similar to the BVDV epidemics in cattle in other regions of China.
Conclusions
This study provides data for monitoring and vaccination strategies of BVDV in western China.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-021-02747-7.
Keywords: BVDV, RT-PCR, Genotype, Bovine, Western China
Background
Bovine viral diarrhea virus (BVDV) is an important pathogen of cattle worldwide and causes significant economic losses. BVDV has been detected in not only in cattle, but also in diverse domestic [1, 2] and wildlife animal species [3–5].
BVDV is a member of the Pestivirus genus within the family Flaviviridae. There are two common BVDV genetic species, BVDV-1 and BVDV-2. However, a newly recognized pestivirus species, “HoBi-like” or “atypical pestiviruses” has been considered as the third genetic species of BVDV [6, 7], and entitled as BVDV-3.The BVDV genome consists of a single-stranded, plus-sense RNA approximately 12.3 kb in length, which is flanked by 5′and 3′untranslated regions (5’UTR, 3’UTR) and encodes 11–12 structural and non-structural proteins (Npro, C, Erns, E1, E2, P7, NS2/3, NS4A, NS4B, NS5A, NS4B). BVDV can be divided into two biotypes, cytopathic (CP) and noncytopathic (NCP), based on its ability of the production of the visible effects on cell culture [8]. The 5’UTR region has primarily been used for subgenotype identification as well as Npro and E2 regions [7, 9–15]. On the basis of the 5’UTR, various subgenotypes of BVDV isolates have been identified. To date, 21 subgenotypes within the BVDV1 (1a-1u) and 3 subgenotypes of BVDV2 have been reported worldwide [10, 14, 16], which predominate in different countries. In China, nine subgenotypes have been identified in cattle, including 1a, 1b, 1c, 1d, 1 m, 1o, 1p, 1q, and 1u [17, 18].
Previous studies showed that a high proportion of BVDV-positive cattle came from western China [19, 20], because these areas are historically the main regions with cattle production. However, studies regarding the genetic diversity of BVDV in western China remain rare [4, 21, 22]. Thus, this study detected and genotyped BVDV from bovines in this region. Such studies are important to understand the diversity of viral strains present in one region and this, in turn, can inform control programs, drive vaccine development and determine likely infection sources.
Results
Detection of BVDV in clinical serum samples
A total of 1234 serum samples were tested for BVDV by 5’UTR RT-PCR. As shown in Table 1, the average positive rate of BVDV in animals was 7.2% (89/1234). At the herd level, a herd was considered positive if one serum sample was positive in RT-PCR testing. Thus, 82.4% (14/17) of herds were positive and they were located in Shaanxi, Ningxia, Xinjiang, and Tibet. No BVDV infection was found in 3 herds from Shaanxi and Ningxia Provinces. The average positive rate of BVDV in Shaanxi, Ningxia, Xinjiang, and Tibet was 4.57% (44/963), 30% (18/60), 37.5% (6/16) and 10.77% (21/195), respectively.
Table 1.
Samples collected and RT-PCR detection of BVDV
| Herd No. | Provinces | Species | Date | Clinical symptoms | No. sample collected | No. positive sample | Positive rate in sample | Status in herd | Positive rate in province |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Shaanxi | Dairy | 2019.3 | Diarrhea | 45 | 0 | 0(0/45) | – | 4.57%(44/963) |
| 2 | Beef | 2019.4 | Diarrhea | 47 | 0 | 0(0/47) | – | ||
| 3 | Diary | 2019.5 | Healthy | 137 | 5 | 3.65%(5/137) | + | ||
| 4 | Diary | 2019.5 | Diarrhea | 29 | 2 | 6.90%(2/29) | + | ||
| 5 | Beef | 2019.5 | Healthy | 211 | 10 | 4.74%(10/211) | + | ||
| 6 | Diary | 2019.5 | Diarrhea | 43 | 8 | 18.60%(8/43) | + | ||
| 7 | Beef | 2019.6 | Healthy | 116 | 9 | 7.76%(9/116) | + | ||
| 8 | Beef | 2019.6 | Diarrhea | 25 | 1 | 4%(1/25) | + | ||
| 9 | Diary | 2019.12 | Diarrhea | 280 | 7 | 2.50%(7/280) | + | ||
| 10 | Diary | 2019.12 | Diarrhea | 30 | 2 | 6.67%(2/30) | + | ||
| 11 | Ningxia | Diary | 2019.4 | Diarrhea | 13 | 2 | 15.38%(2/13) | + | 30%(18/60) |
| 12 | Diary | 2019.4 | Diarrhea | 10 | 6 | 60%(6/10) | + | ||
| 13 | Beef | 2019.4 | Diarrhea | 15 | 10 | 66.67%(10/15) | + | ||
| 14 | Diary | 2019.9 | Diarrhea | 22 | 0 | 0(0/22) | – | ||
| 15 | Xinjiang | Diary | 2019.8 | Diarrhea | 16 | 6 | 37.50%(6/16) | + | 37.5%(6/16) |
| 16 | Tibet | Yak | 2019.7 | Diarrhea | 98 | 15 | 15.31%(15/98) | + | 10.77%(21/195) |
| 17 | Yak | 2019.9 | Diarrhea | 97 | 6 | 6.19%(6/97) | + | ||
| 1234 | 89 | 7.2%(89/1234) | a82.4%(14/17) |
a Rate of positive herds among all the tested herds
Virus isolation
BVDV strains were isolated from RT-PCR positive clinical samples and they were identified by immunofluorescence, RT-PCR and sequence analysis, and transmission electron microscopy.
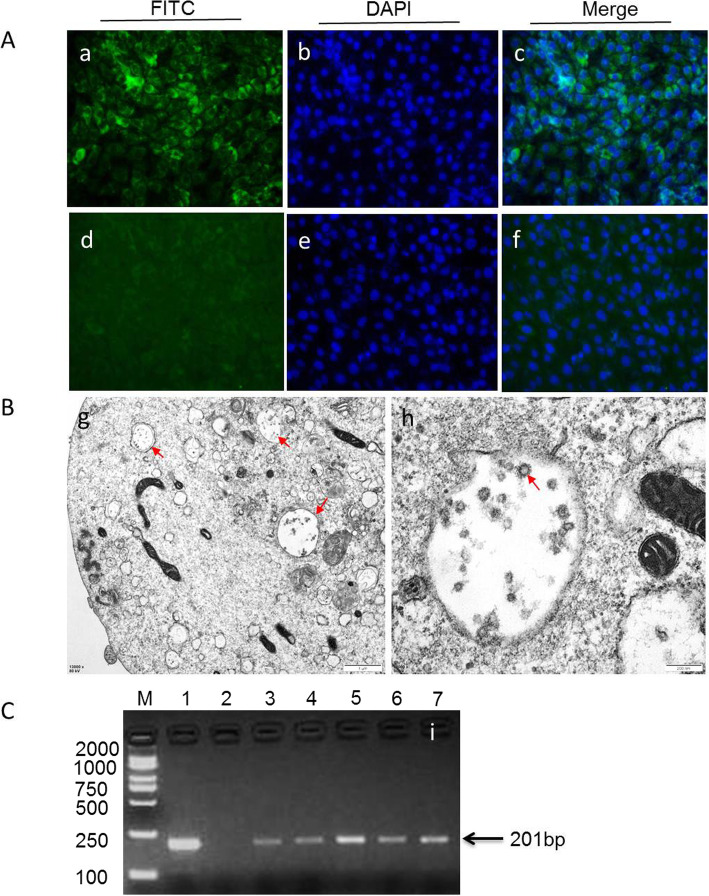
Initially all of the 89 positive samples were subjected to virus isolation, but only 13 BVDV strains were successfully isolated (Fig. 1a-c; S1 Figure). The isolated BVDV strains were named as SX-01XN19, SX-02XN19, NX-05XN19, NX-69209, NX-03XN19, NX-59181, NX-59211, NX-04XN19, NX-10XN19, XJ-06XN19, XJ-07XN19, XJ-08XN19 and XZ-09XN19, respectively. All the 13 strains caused no obvious cell lesions, hence, they were identified as NCP biotype (Fig. 1a). Transmission electron microscopy examination showed typical viral particles in the cytoplasm of MDBK cell, which were measured approximately 60 nm in diameter and occurred as clusters inside vesicles (Fig. 1b). The information of these BVDV strains including source, biotype, and genotype was presented in Table 2.
Fig. 1.
The isolated BVDV strains were confirmed by immunofluorescence, RT-PCR and transmission electron microscope, respectively. a BVDV-infected (a-c) or negative control MDBK cells (d-f) were examined by immunofluorescence using polyclonal antibodies against BVDV E2 protein. b The typical viral particles in the cytoplasm of infected MDBK cell(g, red arrow). The viral particles were measured approximately 60 nm in diameter and occurred as clusters inside vesicles(h, red arrow). c The BVDV strains were detected by 5’UTR RT-PCR (201 bp). Representative electron microscopic image of field BVDV isolates (i). lane M:weight size marker (2000 bp,1000 bp, 750 bp, 500 bp, 250 bp,100 bp), lane 1: positive control; lane 2: negative control, lanes 3–7: BVDV strains isolated from clinical serum samples
Table 2.
List of field virus isolates used in the study
| Virus isolate | Herd | Material | Origin | Genotype | Biotype | GenBank accession no. |
|---|---|---|---|---|---|---|
| SX-02XN19 | 3 | Cell culture | Shaanxi | 1a | ncp | MT316318 |
| SX-2 | 3 | Serum | Shaanxi | 1a | a- | MW142339 |
| SX-1-2 | 4 | Serum | Shaanxi | 1a | – | MW142341 |
| SX-01XN19 | 5 | Cell culture | Shaanxi | 1c | ncp | MT316327 |
| SX-3 | 6 | Serum | Shaanxi | 1a | – | MW142340 |
| SX-219 | 7 | Serum | Shaanxi | 1a | – | MW142338 |
| SX-1-1 | 8 | Serum | Shaanxi | 1a | – | MW142343 |
| SX-3-46 | 9 | Serum | Shaanxi | 1a | – | MW142342 |
| SX-3-2 | 9 | Serum | Shaanxi | 1a | – | MW142344 |
| SX-3-53 | 10 | Serum | Shaanxi | 1a | – | MW142345 |
| NX-69209 | 11 | Cell culture | Ningxia | 1a | ncp | MW142337 |
| NX-03XN19 | 12 | Cell culture | Ningxia | 1c | ncp | MT316319 |
| NX-59181 | 12 | Cell culture | Ningxia | 1c | ncp | MW142336 |
| NX-59211 | 12 | Cell culture | Ningxia | 1c | ncp | MW142335 |
| NX-04XN19 | 12 | Cell culture | Ningxia | 1c | ncp | MT316320 |
| NX-10XN19 | 12 | Cell culture | Ningxia | 1c | ncp | MT316325 |
| NX-05XN19 | 13 | Cell culture | Ningxia | 1a | ncp | MT316321 |
| XJ-06XN19 | 15 | Cell culture | Xinjiang | 1a | ncp | MT316322 |
| XJ-07XN19 | 15 | Cell culture | Xinjiang | 1a | ncp | MT316323 |
| XJ-08XN19 | 15 | Cell culture | Xinjiang | 1a | ncp | MT316324 |
| XZ-N24 | 16 | Serum | Tibet | 1a | – | MW142346 |
| XZ-09XN19 | 17 | Cell culture | Tibet |
1c C |
ncp | MT316328 |
aThe sample was not successfully isolated from cell culture
Sequencing and phylogenetic analysis
To investigate the extent of genetic diversity of BVDV in western China, the subgenotypes of the BVDV isolates were determined by 5’UTR sequencing and phylogenetic analysis.
As shown in Tables 2, 22 virus isolates, including 13 isolated strains and 9 positive serum samples, were randomly selected from 14 BVDV positive herds and used for phylogenetic analysis. BLAST search revealed that all sequences belonged to BVDV-1. As shown in Fig. 2, phylogenetic analysis revealed that these isolates clustered into either BVDV-1a (n = 14) or BVDV-1c (n = 8) subgenotypes. The BVDV-1a subgenotypes were located in Shaanxi (n = 9), Ningxia (n = 1), Xinjiang (n = 3) and Tibet (n = 1), and the BVDV-1c located in Shaanxi (n = 1), Ningxia (n = 6) and Tibet (n = 1).
Fig. 2.
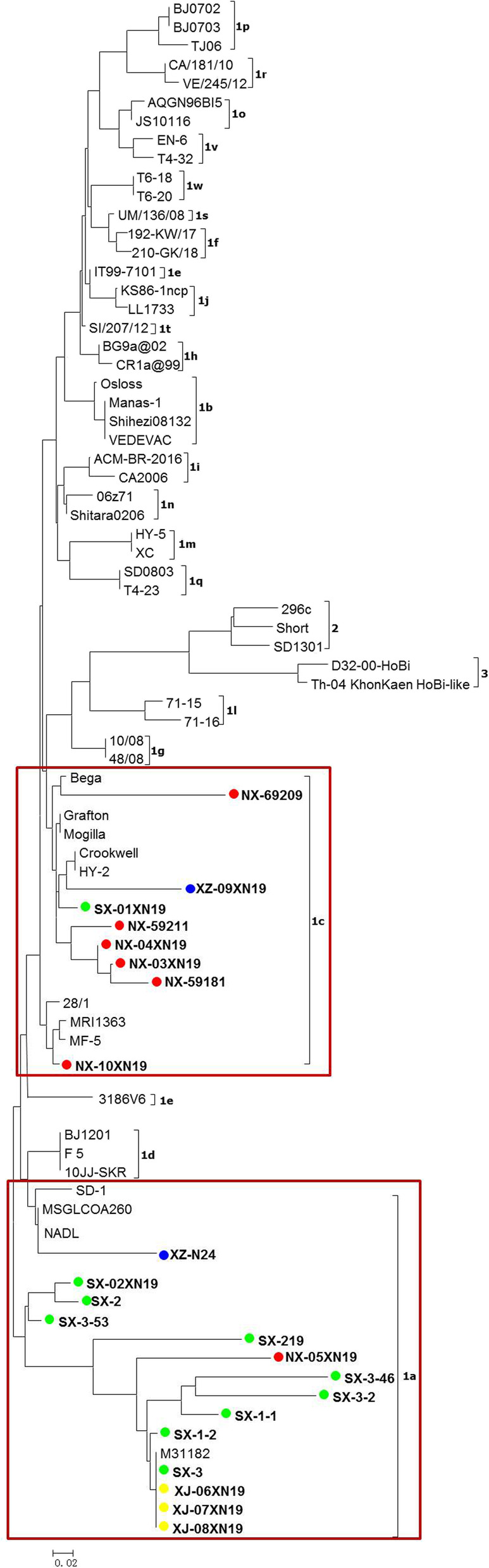
Phylogenetic analysis based on 5’UTR sequence. A phylogenetic tree of the 5’UTR was created using the 5’UTR sequences of 22 selected BVDV isolates and 57 reference isolates retrieved from the GenBank database. ●BVDV isolates identified in provinces of Shaanxi (green dot), Ningxia (red dot), Xinjiang (yellow dot) and Tibet (blue dot) in this study.14 isolates were clustered in BVDV-1a (frame) and 8 isolates clustered in BVDV-1c (frame)
Discussion
In this study, we investigated the prevalence and genetic diversity of BVDV among bovines in western China. The BVDV positive rate varies greatly among bovine herds due to the variety of detection tests, sampling methods, species, and locations. Hence, it is difficult to determine the exact extent of the BVDV prevalence in China. As previously reported, RT-PCR analysis of BVDV RNA is more sensitive than other Ag detection methods and has been widely used for BVDV detection. Primer is pivotal for the accuracy and sensitive of RT-PCR detection. In this study, the primers BP189–389 for 5’UTR region was used, which had a broad range of bovine pestivirus detection including BVDV-1, BVDV-2 and BVDV-3 [23].
Our results demonstrated that the average positive rate of BVDV in animals was 7.2% (89/1234). Previous reports showed that the RNA prevalence of BVDV was 22.64% among bovine in China [17]. A systematic review and meta-analysis showed that the RNA prevalence of BVDV was 27.1% in dairy cattle in China [19]. In our study, there were limitations in the samplings and detection methods. Most of the clinical samples were sent to our laboratory by local farmers without providing detail information on the farms such as size and position. In addition, the sampling site did not completely cover the entire regions in western China. Considering the experimental expenses, the serology test was not performed in this study. Hence, the average RT-PCR positive rate of 7.2% in animals may not reflect the accurate prevalence of BVDV among bovines in western China. Notably, however, at herd level, we found 82.4% (14/17) of herds were positive for BVDV. Recently, a high prevalence of 57.78% of herds was reported to be positive for BVDV in northwestern China [1]. Our results suggested that a high proportion of herds are at risk of BVDV infection in western China.
Virus isolation is the standard method of detection of BVDV-infected cattle. The BVDV infected animals can secrete large amounts of BVDV in their serum, especially persistent infection (PI) animals. Previous studies demonstrated that BVDV remained viable in serum under normal conditions of sample submission to a diagnostic laboratory. Hence, the serum is a valid sample for the isolation of BVDV. In this study, 13 noncytopathic (NCP) strains were successfully isolated from clinical serum samples. NCP biotype is commonly found in nature. Our results further confirmed those of other workers.
BVDV is an RNA virus with high mutation rate [24]. Study investigating the frequency and number of subgenotypes of BVDV is helpful to understand the evolution of the virus and the source of infection. This information also has important implications for the design and construction of effective vaccination strategies to control BVDV [25, 26]. In this study, two subgenotypes of BVDV1a and BVDV1c were identified among 22 selected virus isolates from 14 herds. These results were in agreement with recent epidemiologic studies of BVDV in cattles in China [20, 27]. BVDV2 and BVDV3 were not detected in this study.
Although the predominant subgenotype worldwide is BVDV-1b, BVDV-1a and -1c are the second and third most frequently-reported genotypes in the world, respectively [14].BVDV-1a is predominant in South Africa and is widely circulating in the United States, Korea and Japan [28], while BVDV-1c has been reported as a predominant genotype in Australia [22, 28]. In China, BVDV-1a and -1c have been commonly detected in different regions of China. Recently, BVDV-1c (7/9) and BVDV-1a (1/9) strains were detected from 36 herds of dairy cattle in 5 provinces in eastern China [29]. A recent analysis of 119 BVDV sequences obtained from 92 dairy farms showed that subgenotypes of BVDV-1a (n = 37, 31.09%), BVDV-1c (n = 34, 28.57%) and BVDV-1 m (n = 25, 21.01%) were predominant in 19 provinces of China in 2017 [20].
In western China, scattered studies on the genetic diversity of BVDV among cattle and other ruminants have been reported. Subgenotypes BVDV-1b and BVDV-1c have been identified in cattle from Xinjiang Autonomous Region [22]. BVDV-1b and BVDV-1d were found predominant subgenotypes in dairy farms in Ningxia Autonomous Region [21]. Six subgenotypes of BVDV-1a, BVDV-1b, BVDV-1c, BVDV-1 m, BVDV-1o, BVDV-1p and BVDV-1q have been identified in Bactrian camels from regions of Xinjiang, Gansu and Qinghai [4]. Here, BVDV-1a was respectively detected in Shaanxi, Ningxia, Tibet and Xinjiang, and BVDV-1c was detected in Shaanxi, Ningxia, and Tibet. Taken together, our results confirmed the presence of 1a and 1c subgenotypes in western China. The genetic diversity of virus isolates hamper prevention and control of BVDV. A vaccine effective in one region may fail to protect against virus infection caused by different virus strains in another region [30]. Our findings provide important information for further characterization of the variability and geographical distribution of BVDV in China.
Conclusions
Our results demonstrated that the average positive rate of BVDV was 7.2% (89/1234) in animals and 82.4% (14/17) in herds. Thirteen BVDV strains were successfully isolated from RT-PCR positive clinical samples and they were all NCP biotype. BVDV-1a and 1c subgenotypes were identified from 22 selected isolates from 14 herds. These results confirmed that BVDV-1a and BVDV-1c were circulating in western China, similar to the BVDV epidemics in cattle in other regions of China. This study provides data for monitoring and vaccination strategies of BVDV in western China.
Methods
Clinical sample collection
A total of 1234 serum samples from 17 herds covering 4 provinces in western China (Shaanxi, Ningxia, Xinjiang, and Tibet) were collected in 2019. Most samples were collected from herds in which the animals showed diarrhea. Some samples were submitted from clinical healthy animals for conventional detection. The sample information is summarized in Table 1.These herds were not vaccinated against BVDV. The samples were stored at − 80 °C for RT-PCR and virus isolation.
RT-PCR
The clinical serum samples or cell culture were examined for the presence of BVDV by RT-PCR. Briefly, total RNA was extracted from serum or cell culture using TRIzol Reagent (Gibco). cDNA was synthesized from 1000 ng of total RNA using RNA reverse transcription kit (invitrogen USA).
The synthesized cDNA were submitted sequentially to PCR assay to amplify a 201-bp fragment of the BVDV 5′-UTR region, using referenced primers BP189–389 [21] (Forward: 5′-AGTCGTCAATGGTTCGAC-3′; Reverse: 5′-TCCATGTGCCATGTACA-3′). All PCR reaction were performed in 15 μL volume containing 7.5 μL of 2 × PCR Master Mix (Qiagen), 2 μL of cDNA, 4.5 μL ddH2O, and 10 μM each of the primers. The reaction was carried out at 94 °C for 4 min, followed by 35 cycles of 94 °C for 30 s, 47 °C for 30 s, and 72 °C for 30 s, with a final elongation step of 72 °C for 7 min. The PCR products were checked by electrophoresis on 1% agarose gel.
Virus isolation
To investigate the biotype of BVDV circulating in herds, BVDV strains were isolated from RT-PCR positive clinical samples using standard virus isolation techniques. Briefly, serum samples were placed on Madin-Darby bovine kidney (MDBK) cells for 1 h at 37 °C in a 5% CO2 atmosphere. The cells were washed twice with PBS and then DMEM with 2% fetal bovine serum (BVDV and BVDV antibody-free) was added and incubated for 4–5 days. Then the cultures were frozen and thawed three times and the clarified supernatant was passaged five times in MDBK cells. In the absence of cytopathic effect, the cells were fixed and stained by immunofluorescence as previously described [12]. The supernatants of the infected cells were further tested by RT-PCR described as above for the presence of BVDV nucleic acid.
Transmission electron microscope
The MDBK cell culture infected by RT-PCR positive samples were examined for the presence of BVDV particles by transmission electron microscopy (TEM). Cells were fixed with 2.5% glutaraldehyde in sodium cacodylate buffer (0.2 M, pH 7.2), post-fixed with 1% buffered osmium tetroxide, dehydrated in acetone, and embedded in epoxy resin. The resin blocks were then cut into 60 nm thick ultrathin sections, stained with uranyl acetate and lead citrate, and observed in a TEM (TECNAI G2 SPIRIT BIO).
Sequencing and phylogenetic analysis
The RT-PCR amplified fragments obtained from serum samples or cell culture were directly sequenced by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China). Multiple sequence alignment was performed using the ClustalW program in BioEdit software. Phylogenetic analysis of the 5’UTR region was performed with the neighbor-joining method in MEGA 7.0 software. In the phylogenetic tree, the evolutionary distances were computed using the Tamura 3-parameter model with 1000 bootstrap replicates.
A total of 57 reference sequences of known BVDV-1, BVDV-2 and BVDV-3 isolates were obtained from the NCBI GenBank database (S2 Table). The 22 5’UTR sequences obtained in this study were deposited in GenBank (accession numbers MT316318-MT316325; MT316327-MT316328; MW142335-MW142346), see Table 2.
Supplementary Information
Acknowledgments
Not applicable.
Abbreviations
- BVDV
Bovine viral diarrhea virus
- ORF
Large open reading frame
- RT-PCR
Reverse transcription polymerase chain reaction
- PCR
Polymerase chain reaction
- MDBK
Madin-darby bovine kidney cells
- cDNA
Complementary DNA
- CP
Cytopathogenic
- NCP
Non-cytopathogenic
Authors’ contributions
LLC, XMZ and DWT conceived and designed the experiments. YPQ performed the experiments. LLC wrote the manuscript and analyzed the data. DL contributed to the experiments work. QD contributed to the useful discussion. DWT finalized the manuscript. All authors read and approved the manuscript.
Funding
This study has been supported by the National Nature Science Foundation of China (Grant No. 31972645). The funder had no role in study design, data collection and analysis, interpretation of data or writing of the manuscript.
Availability of data and materials
All the data supporting the results in the current study is contained within the manuscript. Sequences from this study have been deposited in NCBI GenBank under accession numbers as followed: 13 5’UTR sequences from BVDV strains isolated from MDBK cell culture with accession no. MT316318-MT316325, MT316327-MT316328, MW142335-MW142337; 9 5’UTR sequences from clinical serum samples with accession no. MW142338-MW142346.
Ethics approval and consent to participate
Serum samples used in this study had obtained based on informed consent from farm owners. Collection of serum samples from bovines was approved by the Institutional Animal Care and Use Committee (IACUC) of Northwest A&F University (permit numbers 20161112 and 20170516).
Consent for publication
Not applicable.
Competing interests
All authors declared no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lingling Chang and Yanping Qi contributed equally to this work.
Contributor Information
Xiaomin Zhao, Email: xiaominz@nwafu.edu.cn.
Dewen Tong, Email: dwtong@nwsuaf.edu.cn.
References
- 1.Deng Y, Sun CQ, Cao SJ, Lin T, Yuan SS, Zhang HB, Zhai SL, Huang L, Shan TL, Zheng H, Wen XT, Tong GZ. High prevalence of bovine viral diarrhea virus 1 in Chinese swine herds. Vet Microbiol. 2012;159(3–4):490–493. doi: 10.1016/j.vetmic.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Krametter-Froetscher R, Duenser M, Preyler B, Theiner A, Benetka V, Moestl K, Baumgartner W. Pestivirus infection in sheep and goats in West Austria. Vet J. 2010;186(3):342–346. doi: 10.1016/j.tvjl.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Vilcek S, Nettleton PF. Pestiviruses in wild animals. Vet Microbiol. 2006;116(1–3):1–12. doi: 10.1016/j.vetmic.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Gao S, Luo J, Du J, Lang Y, Cong G, Shao J, Lin T, Zhao F, Belak S, Liu L, Chang H, Yin H. Serological and molecular evidence for natural infection of Bactrian camels with multiple subgenotypes of bovine viral diarrhea virus in Western China. Vet Microbiol. 2013;163(1–2):172–176. doi: 10.1016/j.vetmic.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Wang S, Du R, Wang Q, Sun C, Wang N, Zhang P, Zhang L. Isolation and identification of a bovine viral diarrhea virus from sika deer in China. Virol J. 2011;8:83. doi: 10.1186/1743-422X-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauermann FV, Ridpath JF. HoBi-like viruses--the typical 'atypical bovine pestivirus'. Anim Health Res Rev. 2015;16(1):64–69. doi: 10.1017/S146625231500002X. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Romero N, Basurto-Alcantara FJ, Verdugo-Rodriguez A, Bauermann FV, Ridpath JF. Genetic diversity of bovine viral diarrhea virus in cattle from Mexico. J Vet Diagn Investig. 2017;29(3):362–365. doi: 10.1177/1040638717690187. [DOI] [PubMed] [Google Scholar]
- 8.Ammari M, McCarthy FM, Nanduri B, Pinchuk LM. Analysis of Bovine Viral Diarrhea Viruses-infected monocytes: identification of cytopathic and non-cytopathic biotype differences. BMC Bioinformatics. 2010;11(Suppl 6):S9. doi: 10.1186/1471-2105-11-S6-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe Y, Tamura T, Torii S, Wakamori S, Nagai M, Mitsuhashi K, Mine J, Fujimoto Y, Nagashima N, Yoshino F, Sugita Y, Nomura T, Okamatsu M, Kida H, Sakoda Y. Genetic and antigenic characterization of bovine viral diarrhea viruses isolated from cattle in Hokkaido, Japan. J Vet Med Sci. 2016;78(1):61–70. doi: 10.1292/jvms.15-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neill JD, Workman AM, Hesse R, Bai J, Porter EP, Meadors B, Anderson J, Bayles DO, Falkenberg SM. Identification of BVDV2b and 2c subgenotypes in the United States: genetic and antigenic characterization. Virology. 2019;528:19–29. doi: 10.1016/j.virol.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Otonel RA, Alfieri AF, Dezen S, Lunardi M, Headley SA, Alfieri AA. The diversity of BVDV subgenotypes in a vaccinated dairy cattle herd in Brazil. Trop Anim Health Prod. 2014;46(1):87–92. doi: 10.1007/s11250-013-0451-y. [DOI] [PubMed] [Google Scholar]
- 12.Ridpath JF, Bolin SR, Dubovi EJ. Segregation of bovine viral diarrhea virus into genotypes. Virology. 1994;205(1):66–74. doi: 10.1006/viro.1994.1620. [DOI] [PubMed] [Google Scholar]
- 13.Vilcek S, Herring AJ, Herring JA, Nettleton PF, Lowings JP, Paton DJ. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch Virol. 1994;136(3–4):309–323. doi: 10.1007/BF01321060. [DOI] [PubMed] [Google Scholar]
- 14.Yesilbag K, Alpay G, Becher P. Variability and global distribution of subgenotypes of bovine viral diarrhea virus. Viruses. 2017;9(6):128. [DOI] [PMC free article] [PubMed]
- 15.Yilmaz H, Altan E, Ridpath J, Turan N. Genetic diversity and frequency of bovine viral diarrhea virus (BVDV) detected in cattle in Turkey. Comp Immunol Microbiol Infect Dis. 2012;35(5):411–416. doi: 10.1016/j.cimid.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Jenckel M, Hoper D, Schirrmeier H, Reimann I, Goller KV, Hoffmann B, Beer M. Mixed triple: allied viruses in unique recent isolates of highly virulent type 2 bovine viral diarrhea virus detected by deep sequencing. J Virol. 2014;88(12):6983–6992. doi: 10.1128/JVI.00620-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng M, Ji S, Fei W, Raza S, He C, Chen Y, Chen H, Guo A. Prevalence study and genetic typing of bovine viral diarrhea virus (BVDV) in four bovine species in China. PLoS One. 2015;10(4):e0121718. doi: 10.1371/journal.pone.0121718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue F, Zhu YM, Li J, Zhu LC, Ren XG, Feng JK, Shi HF, Gao YR. Genotyping of bovine viral diarrhea viruses from cattle in China between 2005 and 2008. Vet Microbiol. 2010;143(2–4):379–383. doi: 10.1016/j.vetmic.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Ran X, Chen X, Ma L, Wen X, Zhai J, Wang M, Tong X, Hou G, Ni H. A systematic review and meta-analysis of the epidemiology of bovine viral diarrhea virus (BVDV) infection in dairy cattle in China. Acta Trop. 2019;190:296–303. doi: 10.1016/j.actatropica.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Deng M, Chen N, Guidarini C, Xu Z, Zhang J, Cai L, Yuan S, Sun Y, Metcalfe L. Prevalence and genetic diversity of bovine viral diarrhea virus in dairy herds of China. Vet Microbiol. 2020;242:108565. doi: 10.1016/j.vetmic.2019.108565. [DOI] [PubMed] [Google Scholar]
- 21.Gong X, Cao X, Zheng F, Chen Q, Zhou J, Yin H, Liu L, Cai X. Identification and characterization of a novel subgenotype of bovine viral diarrhea virus isolated from dairy cattle in northwestern China. Virus Genes. 2013;46(2):375–376. doi: 10.1007/s11262-012-0861-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhong F, Li N, Huang X, Guo Y, Chen H, Wang X, Shi C, Zhang X. Genetic typing and epidemiologic observation of bovine viral diarrhea virus in Western China. Virus Genes. 2011;42(2):204–207. doi: 10.1007/s11262-010-0558-4. [DOI] [PubMed] [Google Scholar]
- 23.Monteiro FL, Cargnelutti JF, Martins B, Noll JG, Weiblen R, Flores EF. Detection of bovine pestiviruses in sera of beef calves by a RT-PCR based on a newly designed set of pan-bovine pestivirus primers. J Vet Diagn Investig. 2019;31(2):255–258. doi: 10.1177/1040638719826299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzzago C, Ebranati E, Sassera D, Lo Presti A, Lauzi S, Gabanelli E, Ciccozzi M, Zehender G. Spatialand temporal reconstruction of bovine viral diarrhea virus genotype 1 dispersion in Italy. Infect Genet Evol. 2012;122:324–331. doi: 10.1016/j.meegid.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Bachofen C, Stalder H, Braun U, Hilbe M, Ehrensperger F, Peterhans E. Coexistence of genetically and antigenically diverse bovine viral diarrhoea viruses in an endemic situation. Vet Microbiol. 2008;131:93–102. doi: 10.1016/j.vetmic.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Ridpath JF, Fulton RW, Kirkland PD, Neill JD. Prevalence and antigenic diffrences observed between bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the southwestern United States. J Vet Diagn Investig. 2010;22:184–191. doi: 10.1177/104063871002200203. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Shi X, Tong Q, Wu Y, Xia MQ, Ji Y, Xue W, Wu H. A bovine viral diarrhea virus type 1a strain in China: isolation, identification, and experimental infection in calves. Virol J. 2014;11:8. doi: 10.1186/1743-422X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalaycioglu AT. Bovine viral diarrhoea virus (BVDV) diversity and vaccination. A review. Vet Q. 2007;29(2):60–67. doi: 10.1080/01652176.2007.9695228. [DOI] [PubMed] [Google Scholar]
- 29.Hou P, Zhao G, Wang H, He H. Prevalence of bovine viral diarrhea virus in dairy cattle herds in eastern China. Trop Anim Health Prod. 2019;51(4):791–798. doi: 10.1007/s11250-018-1751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridpath J. Preventive strategy for BVDV infection in North America. Jpn J Vet Res. 2012;60(Suppl):S41–S49. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the results in the current study is contained within the manuscript. Sequences from this study have been deposited in NCBI GenBank under accession numbers as followed: 13 5’UTR sequences from BVDV strains isolated from MDBK cell culture with accession no. MT316318-MT316325, MT316327-MT316328, MW142335-MW142337; 9 5’UTR sequences from clinical serum samples with accession no. MW142338-MW142346.