Figure 7. Dermal adipocyte-derived cells become myofibroblasts after injury.
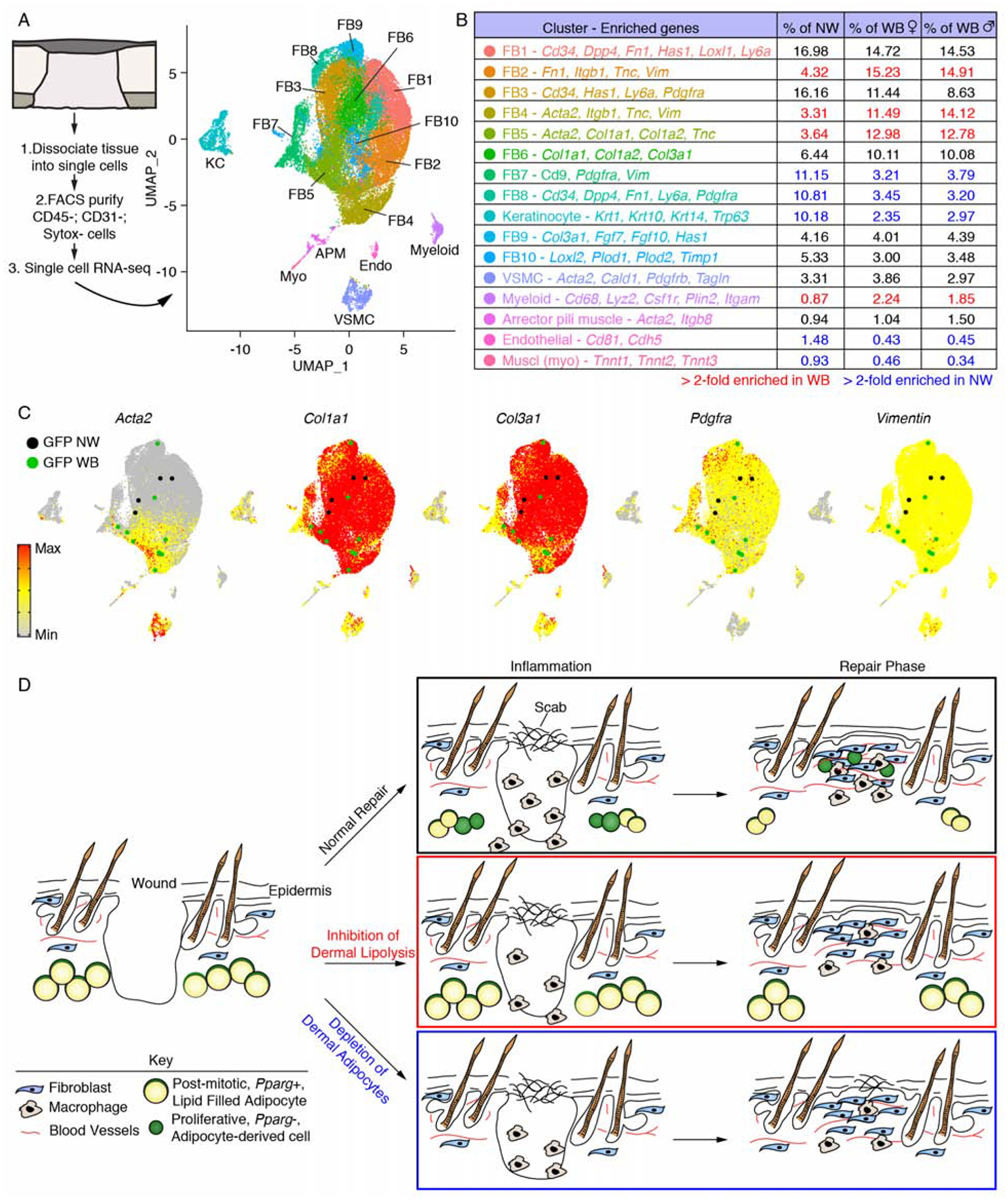
(A) scRNA-seq was performed on non-wounded (NW) skin and day 5 wound beds (WB) from AdipoqCre; mT/mG mice. UMAP dimension reduction plot is displayed with a table (B) identifying cell clusters based on enriched genes and quantifying their relative abundance. (C) Gene expression plots showing distribution of Acta2, Col1a1, Col3a1, Pdgfra and Vimentin. Expression levels for each cell are shown as Pearson residuals and displayed using a color scale, overlaid onto the UMAP plot. GFP+ cells from NW and WB samples are overlaid to emphasize their location. (D) Schematic illustration showing the contribution of adipocytes to skin wound healing. Following injury, adipocytes undergo lipolysis that supports macrophage inflammation. Adipocytes depleted of lipids become myofibroblasts and proliferate. Depletion of dermal adipocytes and inhibition of dermal adipocyte lipolysis reduces macrophage numbers during inflammation and delays revascularization. Ablating dermal adipocytes also delays re-epithelialization. FB, fibroblast; VSMC, vascular smooth muscle cell.
