Abstract
Multidisciplinary research efforts in the field of drug delivery have led to the development of a variety of drug delivery systems (DDS) designed for site-specific delivery of diagnostic and therapeutic agents. Since efficient uptake of drug carriers into target cells is central to effective drug delivery, a comprehensive understanding of the biological pathways for cellular internalization of DDS can facilitate the development of DDS capable of precise tissue targeting and enhanced therapeutic outcomes. Diverse methods have been applied to study the internalization mechanisms responsible for endocytotic uptake of extracellular materials, which are also the principal pathways exploited by many DDS. Chemical inhibitors remain the most commonly used method to explore endocytotic internalization mechanisms, although genetic methods are increasingly accessible and may constitute more specific approaches. This review highlights the molecular basis of internalization pathways most relevant to internalization of DDS, and the principal methods used to study each route. This review also showcases examples of DDS that are internalized by each route, and reviews the general effects of biophysical properties of DDS on the internalization efficiency. Finally, options for intracellular trafficking and targeting of internalized DDS are briefly reviewed, representing an additional opportunity for multi-level targeting to achieve further specificity and therapeutic efficacy.
Keywords: Caveolae-mediated endocytosis, Clathrin-mediated endocytosis, Endocytosis, Imaging, Macropinocytosis, Phagocytosis, Drug delivery, Drug Carrier, CAM-endocytosis
Graphical abstract
1. Introduction
In recent years, the advantages associated with the use of an increasing variety of nanomaterials to formulate new drug delivery systems (DDS) have been demonstrated. Nanomaterials have been incorporated into many experimental DDS due to their ability to improve drug solubility, enable targeting of specific tissues, reduce systemic toxicity and increase cellular uptake of encapsulated or attached drugs at the target site. Knowledge of the cellular uptake pathways and the subsequent intracellular trafficking and disposition of DDS, which enter cells largely by endocytosis, is an important area of investigation in order to maximize the therapeutic effects of encapsulated or attached drugs. Tremendous efforts have been made to understand the cellular uptake mechanisms of DDS, with several reviews and book chapters summarizing the pathways and the factors affecting cellular uptake written by investigators representing pioneers in the field [1–6]. This review will discuss the principal endocytosis pathways relevant to uptake of DDS, and highlight examples of DDS that are targeted to each of these mechanisms. This review also showcases tools and approaches that can be used to further study these pathways and to improve the targeted delivery of new DDS, with a particular emphasis on emerging genetic and in vivo approaches which may enable greater precision in dissecting different endocytotic pathways. This review finally highlights features governing the ultimate bioaccumulation and intracellular distribution of DDS.
2. Principal Mechanisms of Endocytosis
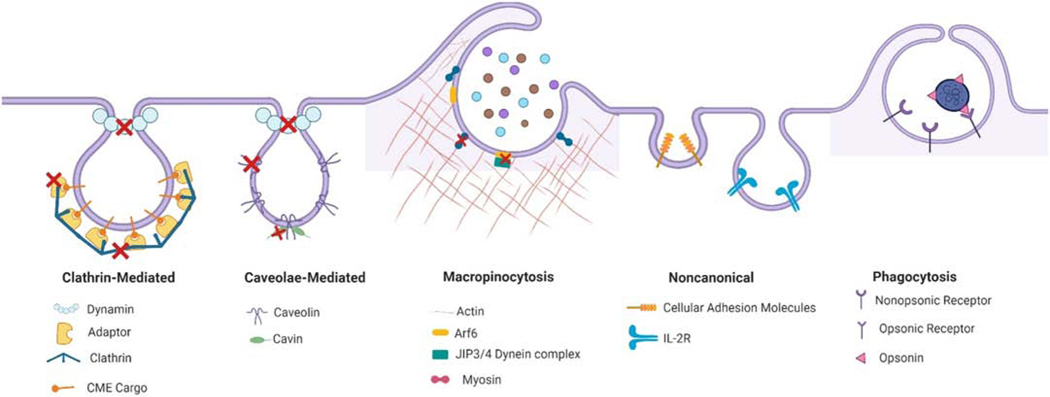
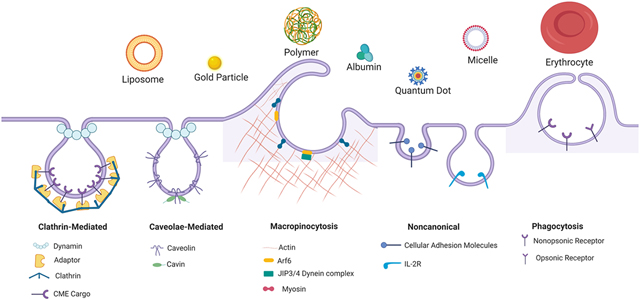
Endocytosis is the process by which cells internalize and transport surface proteins, lipids and other macromolecules enveloped within small membrane vesicles formed by invagination of the plasma membrane into the cell interior. This process is key in the regulation of many essential cellular processes involved in homeostasis and communication including internalization of transmembrane receptors, uptake of extracellular vesicles, plasma membrane remodeling and cell surface signaling [7, 8]. This process takes place through several distinct pathways including clathrin-mediated endocytosis (CME); caveolar endocytosis; cholesterol-sensitive clathrin- and caveolar-independent endocytosis; clathrin independent carriers (CLIC)-glycophosphatidylinositol-anchored protein-enriched endosomal compartments (GEEC) (CLIC/GEEC) endocytosis, non-canonical endocytosis, macropinocytosis and phagocytosis. Here we focus on the 5 principal pathways currently most relevant to uptake of DDS (Figure 1): CME, caveolae-mediated endocytosis, macropinocytosis, phagocytosis and noncanonical endocytosis (specifically CAM-mediated and interleukin receptor 2, (IL-2R)-mediated).
Figure 1. Schematic summary of the cellular uptake mechanisms and genetic targets which have been utilized to manipulate endocytotic pathways involved in drug delivery.
Red crosses mark the genetic targets used to manipulate the different endocytotic pathways. Targeting of clathrin, adaptor proteins and dynamin all affect clathrin-mediated endocytosis. Genetic methods targeting different members of the caveolin or cavin family and dynamin also disrupt caveolaer-mediated endocytosis in different tissues. Targeting of members of the myosin family, JIP3/4, and Arf6 have been shown to interfere with macropinocytosis. Less consensus exists on the role of specific molecular effectors of the other endocytosis pathways indicated as useful for drug delivery. This schematic was created using BioRender.com
2.1. Clathrin-mediated endocytosis.
CME constitutes the principal endocytotic pathway in most mammalian cells. It occurs through a well-orchestrated multistep process involving assembly and maturation of a clathrin coat at the plasma membrane into a ~100 nm coated pit where cargo is concentrated. The pits further invaginate and are finally detached from the plasma membrane by dynamin to form clathrin-coated vesicles. The released vesicles subsequently lose their coat, participate in multiple homotypic fusion events, and eventually deliver their cargo to early sorting endosomes. CME has traditionally been considered as a constitutive process but with the development of advanced live cell imaging techniques, new evidence suggests that CME is highly regulated, with regulators functioning at each step of clathrin-coated pit initiation, cargo selection, vesicle maturation, and fission. Regulation of CME has been reviewed elsewhere [7].
Although orchestrated by a few key proteins including clathrin heavy chain (CHC), clathrin light chains (CLC), and the heterotetrameric adaptor protein-2 (AP-2) complex consisting of α, β2, μ2, and σ2 subunits, the whole CME process involves more than 50 different proteins. These proteins fulfill multiple functions as components of scaffolds, cargo recruiters for specific cargoes, facilitators of membrane curvature as well as regulators of the whole process. During coat formation, cytosolic coat proteins are recruited to a specific region of the plasma membrane. These coat proteins consist of clathrin-adaptor proteins including the AP-2 complex, epsins, clathrin assembly lymphoid myeloid leukemia proteins (CALMs), and scaffold proteins including CHC, CLC, and intersectins, all of which interact to assemble the coat. Many of the coat proteins including the AP-2 complex are also major players in cargo recognition and concentration. As cargo is recruited, the clathrin-coated pit deepens and matures, assisted by naturally curved, (Bin-Amphiphysin-Rvs or BAR) domain-containing proteins that create progressive curvature. In the last step, the large GTPase, dynamin, present in the clathrin-coated pit from the early stages, assembles into helical rings around the neck of mature invaginated pits to facilitate their fission from the plasma membrane. A schematic of this process is shown in Figure 1, highlighting key effectors.
2.1.1. Tools to study CME
2.1.1.1. Chemical inhibitors
Chemical inhibitors of endocytosis and their working mechanisms have been extensively reviewed previously [9, 10]. Some classic chemical inhibitors of CME include methyl-β-cyclodextrin [11], which selectively extracts cholesterol from the plasma membrane thus inhibiting the invagination of clathrin-coated pits; chlorpromazine [12], which prevents coated pit assembly at the cell surface; monodansylcadaverine [13], which disrupts the association of ligand with clathrin-coated pit regions of the plasma membrane; and pitstop [14], which selectively blocks association between clathrin terminal domain and its accessory protein tether to the receptor, have been utilized for decades. These tools, while useful, are plagued by a lack of specificity to particular endocytotic pathways. A summary of classical and newer chemical inhibitor tools used to study endocytosis is found in Table 1.
Table 1.
Pharmacological and Genetic Tools for Manipulation of Endocytosis
| Pathways | Chemical inhibitors | Genetic Targets | Protein Engineering | References |
|---|---|---|---|---|
| Clathrin-mediated | Methyl-β-Cyclodextrin*, Chlorpromazine, Monodansylcadaverine, Pitstop, Tyrphostin A23, Endosidin 9, ES9–17, Ikarugamycin | Clathrin heavy chain, Clathrin light chain, AP-2 μ2 subunit, Epsins, Dynamins | “Hot Wiring”, “Molecular Switch” | [9, 10, 19–27, 29–35, 37–42, 382] |
| Caveolae- mediated | Filipin, Nystatin, Methyl-β-Cyclodextrin | Caveolin family proteins, Cavins, Dynamins | N/A | [9, 10, 35–41, 68, 70–77] |
| Macropinocytosis | EIPA, Wortmannin, Imipramine, Phenoxybenzamine, Vinblastine | Myosins, Ras, PI3 kinase, Src, Rac, Microtubules, Dynein motors, Arf6 effectors, JIP3/JIP4 | N/A | [9, 119–122, 126, 383] |
| CAM-mediated | Amiloride, Bisindolylmaleimide-1,(5-isoquiniline sulphonyl)-2-methylpiperazine, Latrunculin, Radicicol, Y27632 | Protein kinase C, Dynamin, Actin, Src kinase and Rho kinase, Na+/H+ exchanger family proteins | N/A | [194, 202] |
| IL-2R-mediated | Dynasore, Dyngo-4a, Cytochalasin D, Latrunculin A, Jasplakinolide, | Endophilins, Dynamin, Rac, Rho, Phosphatidylinositol-3-OH kinase, PAK1, Actin, Cdc42 | N/A | [209, 210] |
| Phagocytosis | Cytochalasin B, Adenosine, Deoxyadenosine, Adenine arabinoside, Mycotrienin, Piericidin, Genistein, Latrunculin A, Thimerosal and P-nitrophenyl methyl disulfide | RAC1, DOCK2, SCAR/WAVE complex, ARP2/3 complex, mTOR-associated Regulator complex, NHLRC2 | N/A | [139–143, 156] |
Bold: Identified in multiple studies; Not bold: Supported by 1–2 studies
Identification of more specific inhibitors of CME that do not affect other endocytotic pathways, has long been a goal. Banbury et al discovered that Tyrphostin A23, a structural analog of tyrosine, could inhibit the endocytosis of transferrin (Tf) and TGN38, internalization of which are highly dependent on the interaction between the tyrosine based motif in these proteins and AP-2, by inhibiting the interaction between the two [15]. This effect occurred without inhibition of pinocytosis. A decade later, another group proposed that Tyrphostin A23 inhibited CME in Arabidopsis thaliana due to its ability to uncouple oxidation and phosphorylation in mitochondria, thus acidifying the cytoplasm; this acidification caused a dramatic increase in the dwelling time of clathrin and associated adaptors with other effectors, thus inhibiting their normal dynamic behavior and leading to a reduction of phosphatidylinositol 4,5-biphosphate and inhibition of clathrin-coated pit formation [16]. They also discovered that Endosidin 9, a novel mitochondrial uncoupler, could inhibit CME in different systems due to its protonophore activity that resulted in cytoplasmic acidification, similarly to Tyrphostin A23 [16], as well as through direct binding to CHC [17]. At the same time, they identified an improved Endosidin 9 analogue, ES9–17, which lacked the effects of Endosidin 9 on cytoplasmic pH while retaining its ability to target CHC [17]. Elkin et al showed that ikarugamycin, a natural product with anti-protozoal activity, could inhibit CME of the Tf receptor (TfR), low density lipoprotein receptor (LDLR) and epidermal growth factor receptor (EGFR) in various cell lines in a rapid and reversible fashion, although long-term incubation with ikarugamycin was cytotoxic [18].
2.1.1.2. Genetic tools
Clathrin heavy and light chain modifications:
To avoid problems associated with low selectivity of chemical inhibitors, genetic approaches have also been applied to inhibit or induce endocytosis (Table 1). One major strategy is to alter the expression or function of endocytosis-related proteins in vitro. Genetically modified animal models also provide an additional approach to study endocytosis in vivo, thus enabling greater understanding of endocytosis mechanisms within a three-dimensional multicellular environment. Knockdown of CHC is one of the most effective strategies to prevent CME. siRNA knockdown of CHC in a HeLa cell monolayer resulted in lack of detection of clathrin-coated pits or vesicles concurrent with swelling of post-Golgi membrane compartments. Receptor-mediated endocytosis of TfR, LDLR and EGFR chimeras were severely inhibited in clathrin-depleted cells [19]. In contrast, knockdown of CLC did not significantly influence the endocytosis of classical cargoes such as Tf and epidermal growth factor (EGF) [20], but was able to alter endocytosis of specific cargoes such as some G-protein coupled receptors (GPCR) [21] and bacteria [22]. Interestingly, knockdown of CLC significantly impacted clathrin-mediated trafficking between the trans-Golgi network and the endosomal system through additional downstream actions on actin polymerization, resulting in fission of clathrin-coated pits through interaction with myosin VI at the apical surface, and also impacted cell migration through interference with recycling of β1-integrin [23–25].
Deletion of CHC in Caenorhabditis elegans resulted in strongly inhibited endocytosis of yolk by oocytes and development of inviable embryos [26]. In Trypanosoma brucei, depletion of CHC by RNA interference (RNAi) caused dramatically reduced endocytosis and growth cessation, as well as development of an enlarged flagellar pocket due to the coalescence of many intracellular vesicles [27]. Recently, using CRISPR technology, the Jackson Laboratory has successfully developed a mouse expressing a mutant CHC, termed B6N;FVB-Cltcem1(IMPC)J/Mmjax [28] which has the potential to become an important tool in future studies of CME. Interestingly, B-cells derived from CLC isotype aknockout mice showed decreased endocytosis of transforming growth factor β receptor 2, CXCR4, and δ-opioid receptor, but not CXCR5 or β2-adrenergic receptor, reinforcing the possible role of CLC in guiding cargo selectivity in vivo [29].
Modifications of adaptor proteins and other CME effectors:
AP-2 μ2 subunit depletion by siRNA in HeLa cell monolayers significantly inhibited the formation of clathrin-coated pits associated with the plasma membrane, although it did not completely eliminate them. Endocytosis of TfR, but not EGFR or LDLR, was severely reduced in AP-2 depleted cells, suggesting that AP-2 depletion impacted CME in a cargo-dependent way [19], similar to CLC. Interestingly, no AP-2 μ2 homozygous mutant mice embryos were identified among blastocysts from intercrossed heterozygotes, indicating that μ2-deficient embryos die before day 3.5 postcoitus. AP-2 μ2 heterozygous mutant mice were viable and exhibited an apparently normal phenotype. Endocytosis of the plasma membrane pool of CD63, which is endocytosed through AP-2 mediated mechanisms, was not affected in embryonic fibroblasts from AP-2 μ2 heterozygous mutant mice [30].
siRNA knockdown of Epsin1 in BS-C-1 cells did not affect the entry of several classical ligands for CME including Tf, LDL or EGF, but significantly inhibited CME of influenza virus [31]. Interestingly, siRNA knockdown of both Epsin 1 and Epsin 2 in human umbilical vein endothelial cells (HUVEC) cells significantly reduced the endocytosis of vascular endothelial growth factor receptor (VEGFR)-1. Epsin 1 and Epsin 2 double knockout mice showed embryonical lethality at around embryonic day 10; however, embryonic fibroblasts from double knockout mice did not show obvious differences in clathrin-coated pit formation or endocytosis of Tf and EGF. Interestingly, endothelial cell derived from mice with deletions of Epsin 1 and Epsin 2 in vascular endothelium also showed defective endocytosis and subsequent degradation of VEGFR2 [32]. Simultaneous depletion of Epsin 1, 2, and 3 led to a significant decrease in Tf uptake, which was attributed to a defect in the scission of clathrin-coated vesicles without interrupting dynamin [33]. In accord with this finding, mouse embryonic fibroblasts from Epsin 1, 2,and 3 triple knockout mice also showed significantly reduced uptake of Tf in parallel with accumulation of early U-shaped pits [34].
Modification of Dynamins:
Dynamin is a critical effector of both CME and caveolae-dependent endocytosis (discussed below). There are three isoforms of dynamin: dynamin-1, dynamin-2 and dynamin-3. Dynamin-1 is specific to the central nervous system, and highly enriched in presynaptic nerve terminals. Dynamin-2 is ubiquitously expressed in all tissues. Dynamin-3 is predominantly expressed in the testis and in some regions of the brain. Although caveolae-dependent endocytosis is dynamin-dependent as well, most studies have applied genetic approaches to the analysis of CME. Dominant-negative mutants of dynamin-1 and dynamin-2, dyn1(K44A) and dyn2(K44A), significantly inhibited the endocytosis of Tf in HeLa cells with minimal effects on other membrane trafficking events [35]. Unexpectedly, when dynamin mutants defective in self-assembly-stimulated GTPase activity were overexpressed, namely dyn(K694A) and dyn(R725A) mutants, CME was accelerated [36]. A follow-up study showed that these mutations accelerated the formation of constricted coated pits, known to be the rate limiting step in endocytosis [37]. Neurons from dynamin-1 knockout mice exhibited impaired fission of clathrin-coated vesicles, manifested as a strikingly higher accumulation of clathrin-coated buds attached to the plasma membrane at the synapse compared with cells from wild type mice [38]. Dynamin-2 conditional knockout cells showed significantly impaired CME, p75 export from the Golgi, platelet-derived growth factor-stimulated macropinocytosis and cytokinesis, but had no effects on other endocytotic pathways [39]. Similarly, cells derived from dynamin-1/dynamin-2 double knockout mice showed impaired maturation of clathrin-coated pits into free clathrin coated vesicles [40]. Dynamin-1/−2/−3 triple knockout cells did not reveal any additional defects beyond the dynamin-1/−2 double knockout cells [41].
Protein engineering approaches for regulation of CME:
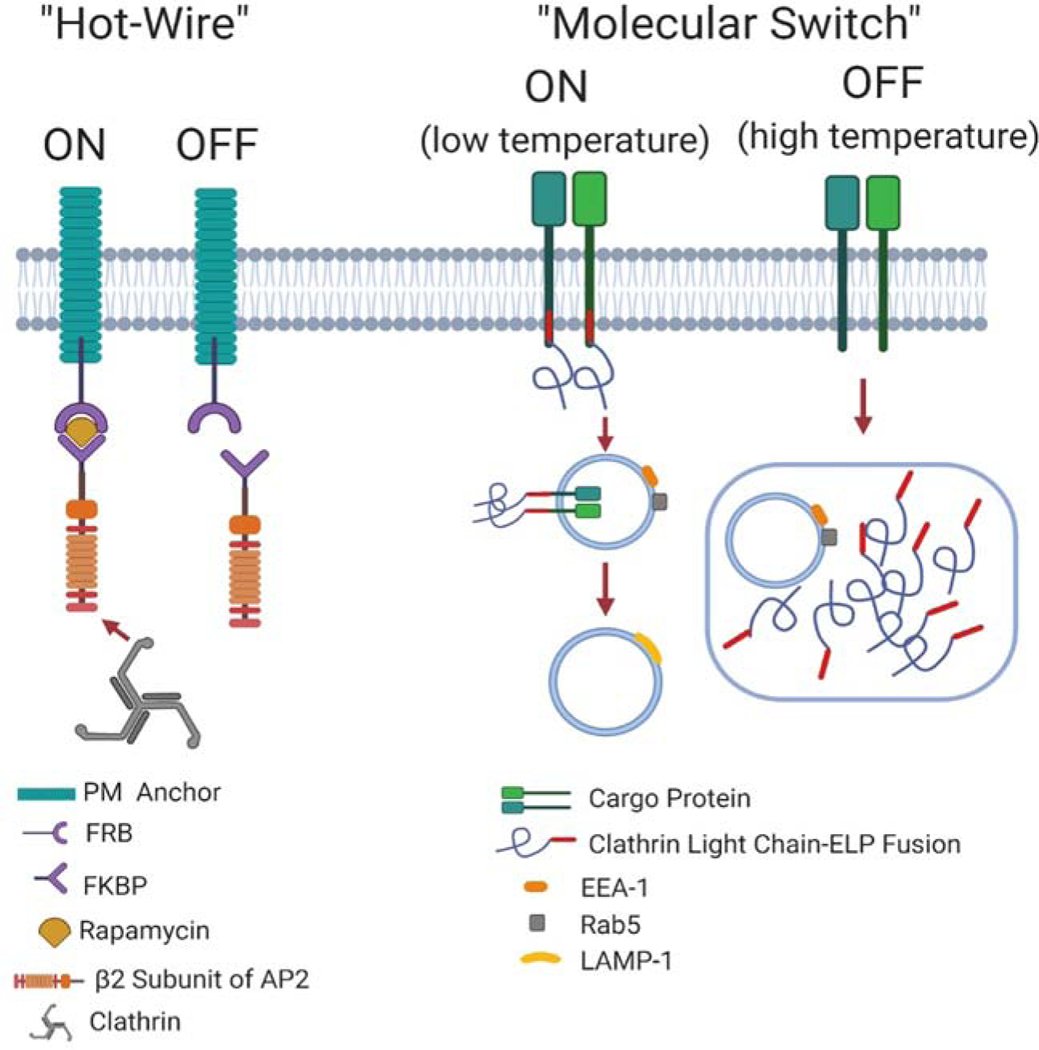
While CME can be inhibited by genetic methods through deletion or mutant expression of its key effectors, activating CME on demand is not as straightforward. Wood et al. developed a series of genetically encoded reporters that can initiate CME through chemical induction. A clathrin-binding fragment of AP2 (“hook”) is fused to the FK506-binding protein which can bind rapamycin. The “anchor” protein at the plasma membrane is likewise fused to another rapamycin-binding protein. Addition of rapamycin triggers recruitment of the AP2 hook to the anchor protein of interest, subsequently recruiting clathrin and initiating CME (Figure 2). The authors termed this system “hot-wiring” because several intermediate steps in vesicle creation were bypassed. This “hot-wiring” system is useful for understanding which factors are essential for the initiation of endocytotic events as well as for studying the downstream processing of hot-wired vesicles and the machineries that control these aspects of vesicle trafficking [42].
Figure 2. Schematic illustration of protein engineering methods used to study CME.
For the “Hot-Wire” method, CME is turned on in the presence of rapamycin. The FK506 binding protein (FKBP)-AP2 complex can be recruited to the plasma membrane in the presence of rapamycin through binding to rapamycin binding protein (FRB) which is anchored to the plasma membrane through fusion to a plasma membrane protein. Clathrin can be further recruited to initiate CME. In the absence of rapamycin, the FKBP-AP2 complex cannot bind to FRB. Thus, clathrin will not be recruited to the plasma membrane and CME is turned off. For the ”Molecular Switch ”method, at low temperature, CLC-ELP remains soluble and maintains normal CME function. At high temperature, CLC-ELP forms microdomains and shuts off CME. This schematic was created using BioRender.com
Pastuczka et al. reported a temperature responsive method to reversibly turn on and off CME on demand, i.e. the “molecular switch”. CLC was fused to thermo-responsive Elastin-Like Polypeptides (ELPs) which can be assembled into microdomains in the cytosol triggered by a temperature increase (Figure 2). These organelle-sized microdomains, which can be thermally activated and inactivated within minutes, are reversible, do not require exogenous chemical stimulation, and are specific for components trafficked within the CME pathway [43]. Upon formation of these microdomains, internalization of angiotensin II receptor which is dependent on CLC was inhibited. Compared with genetic methods that knock down critical effectors of CME, the advantage of this technique is that it is reversible and can be evaluated in healthy cells simply transfected with CLC-ELP, which have not been passaged under conditions of constitutive inhibition of CME that may cause secondary effects [43]. However, as mentioned above, knockdown of CLC only selectively inhibits the endocytosis of certain CLC-dependent cargoes; therefore, this “molecular switch” can only reversibly turn “on” and “off” the CME of CLC-dependent cargoes, such as angiotensin II receptor and some other GPCR. The effects on other CLC-independent cargoes have not yet been investigated so it is unclear whether the differential sensitivity to CLC knockout exhibited by some receptors will be similarly observed with this approach.
2.1.2. CME in drug delivery
TfR-targeted DDS are by far the most widely used drug delivery approaches that take advantage of CME to achieve intracellular delivery [44]. Different strategies such as direct conjugation of Tf with the active drug, direct conjugation of anti-TfR antibody or a TfR-targeting peptide with the active drug, or conjugation of Tf with DDS encapsulating various drugs have all been extensively applied in preclinical models for targeting of cancers and the central nervous system [45, 46]. Studies have shown that the cellular uptake efficiency of nanoparticles increases with increasing density of Tf or anti-TfR antibodies on the nanoparticle surface [47, 48]. While this enhances uptake, a noticeably, higher surface density of Tf or of TfR antibodies can also elicit higher cytotoxicity, which indicates that the surface density of the targeting moiety should be carefully adjusted when using TfR-targeted DDS [47].
Clinical trials exploring TfR-targeted delivery of biofunctional materials have been ongoing since the 1990s; however, so far there has been no FDA-approved therapy based on TfR-targeted drug delivery. Several Tf-drug conjugates have transitioned from preclinical efforts into clinical trials, including Tf-CRM107 (modified diphtheria toxin), a conjugate which showed promising therapeutic effects in patients with refractory glioblastoma multiforme or anaplastic astrocytoma in Phase II clinical trials [49]. Tf-cisplatin conjugates also showed promising effects in breast cancer patients in a Phase I clinical trial [50]. However, no further reports were available from these trials.
Some new strategies for TfR targeting such as use of Tf-liposome conjugates have also drawn attention. MBP-426, a liposome conjugated with Tf for the delivery of oxaliplatin in patients with gastric, gastroesophageal, or esophageal adenocarcinomas, showed good tolerance with minimal toxicity in a Phase I clinical trial [51]. SGT-53, a liposome encapsulating plasmid DNA coding for wild type p53 conjugated with anti-TfR single chain variable fragment showed satisfactory tolerance and promising therapeutic effects in patients with advanced solid tumors in a Phase Ib clinical trial [52]. SGT-94a, an anti-TfR single chain variable fragment-conjugated liposome encapsulating RB94 plasmid, also showed good tolerance in metastatic genitourinary cancer patients in a Phase I clinical trial [53]. Additionally, a cyclodextrin-based polymer conjugated with Tf and loaded with siRNA targeted to reduce the expression of ribonucleotide reductase has shown evidence for specific gene inhibition in humans in an ongoing Phase I clinical trial [54]. Like TfR-drug conjugates, these efforts have not progressed to Phase III trials due to adverse events and systemic toxicity as reported in the Phase I or II clinical trials. The enhanced toxicity of TfR-targeted delivery seen both for drug conjugates and drug carriers may be caused by the TfR targeting moiety itself, through disruption of iron metabolism of the body, or through the increased targeting of cytotoxic drugs to non-target tissues also expressing TfR. More mechanistic studies are needed to elucidate the precise cause of toxicity before these delivery strategies can be further advanced.
EGF and/or anti-EGFR antibody-conjugated DDS are another example of approaches intended to achieve intracellular delivery of bifunctional molecules through CME. These strategies have been widely explored in cancer-targeted therapy. Aerosol delivery of EGF-modified gelatin nanoparticle carriers loaded with cisplatin showed enhanced delivery to the lungs in a lung cancer mouse model, suggesting possible benefit in clinical treatment for lung cancer [55]. To avoid introducing exogenous EGF with its known mitogenic properties, the anti-EGFR antibody has been more widely used as a targeting moiety for EGFR. Various anti-EGFR antibody-conjugated drug carriers loaded with chemotherapeutic drugs have showed significant therapeutic effects in mouse cancer models over the years [56–58]. Currently, one EGFR-targeted therapy, an anti-EGFR-antibody modified immunoliposome loaded with Doxorubicin, is in clinical trials for triple negative breast cancer (NCT02833766, Phase II) and refractory high-grade glioma (NCT03603379, Phase I).
LDLR-targeted drug delivery, GPCR-targeted drug delivery, vascular cell adhesion molecule (VCAM)-1 targeted drug delivery and Integrin-targeted drug delivery are other examples of targeting approaches that have targeted internalization via CME to deliver drugs, radionuclides, proteins, and nucleic acid intracellularly to achieve diagnostic or therapeutic goals [59]. Examples using these delivery strategies are summarized in Table 2. However, the majority of these studies are still in preclinical animal models.
Table 2.
Targeted drug and DDS thought to be internalized by CME
| Target | Ligand | Examples of Conjugated Moieties explored in preclinical studies | Potential clinical Applications | Referen ces |
|---|---|---|---|---|
| TfR | Tf, anti-TfR Ab, TfRscF, TfR- targeted peptide | Drugs, toxic protein, enzyme, nucleic acid, polymers, micelles, liposomes, cyclodextrin, recombinant viral vectors | Cancer therapy, Gene Ther Drug delivery to CNS | [44, 45] |
| EGFR | EGF, anti-EGFR Ab, Fab of anti-EGFR Ab, EGFRsc Fv, | Liposomes, polymers, superparamagnetic iron oxide nanoparticles, gelatin nanoparticles, carbon nanodiamonds, gold nanoparticles, Albumin | Cancer therapy | [55, 57–59, 384] |
| LDLR | LDL, LDLR targeting peptide, | siRNA, liposomes, near-infrared dyes | Cancer therapy, Gene Ther, Cancer imaging | [385–387] |
| GPCR | Targeting ligand, agonist, antagoni st |
Radioisotopes, quantum dots | Cancer therapy | [388, 389] |
| Integrin | Targeting peptide, small molecule s,Ab | Drugs, liposomes, graphene and graphene oxide nanoparticles, silica nanoparticles, chitosan nanoparticles, ferritin, polypeptides, albumin nanoparticle, adenovirus vectors | Cancer therapy, Cancer imaging, Gene Ther | [390] |
| VCAM-1 | Anti-VCAM-1 Ab, targeting peptide, | Liposomes, gold particles, core-shell nanoparticles, lipid nanoemulsions | Cancer therapy, Gene Ther, antiinflammation, CNS delivery | [391] |
2.2. Caveolar endocytosis
Caveolae are uncoated omega-shaped pits of 50–100 nm in diameter located at the plasma membrane in mammalian cells. Distinct from the process of CME which involves a dynamic and sequential maturation, caveolae appear to be consistently detected in a defined shape with consistent curvature and proportions in the neck region [60]. They may also form clusters or rosettes of several caveolae which are open to the plasma membrane through an intermediate single neck [61]. The density of caveolae varies between different cell types and tissues, but they may double the cell surface area in certain cell types. The proteins involved in caveolar endocytosis can generally be divided into structural and accessory proteins [60]. The structural proteins include the cholesterol-binding caveolins and the cavin coat proteins, while key accessory proteins include Eps15 Homology Domain (EHD) proteins and pacsin/syndapin. Caveolins and cavins are mostly located in the caveolar bulb while the EHD proteins are primarily located in the neck region of the caveolae [61, 62]. A schematic showing the major effectors for caveolae-mediated endocytosis is shown in Figure 1.
The exact function of the caveolae has been debated, and its endocytotic capabilities and dynamicity questioned. Specifically, there have been conflicting reports on whether caveolae actually bud into endocytic carriers, as well as questions about the relative mobility of these potential carriers. However, with development of genetic knock-in and knock-out models and the application of high-resolution electron and live cell light and fluorescence microscopy, it is generally agreed that caveolae-mediated endocytosis does occur. In addition, several other functions for caveolae have now been suggested including signal transduction, mechanoprotection, and lipid regulation. It is still unclear if these functions occur concurrently in all cells, or if they are cell-type dependent. These expanded roles and their relationship to disease has been recently reviewed [60].
Caveolar endocytosis occurs when external cargo binds to cargo receptors concentrated in the caveolar bud. This binding triggers budding of the caveolae from the plasma membrane, forming an endocytotic caveolar carrier containing both caveolin-1 and cavin-1 [60]. This process, like CME, depends on dynamin-mediated detachment of the vesicle. The caveolar budding process has been reviewed in [63]. The ultimate destination of the budded endocytotic carrier has also been debated. In 2001, Pelkmans et al. suggested the presence of a neutral pH organelle termed the caveosome, which facilitated a direct pathway from the caveolae to the endoplasmic reticulum (ER), bypassing the endosomes [64]. However, in 2010, new work from the same group demonstrated two different trafficking pathways for caveolin-1 that are regulated by the level of its expression [65]. These studies suggested that under constitutive expression conditions, caveolae bud from the plasma membrane carrying caveolin-1 and cavins, and are trafficked to the early endosome. However, when caveolin-1 was highly expressed, it was targeted to endosomes, ubiquitinated, sequestered into intraluminal vesicles by specialized endosomal sorting complexes required for transport proteins, and degraded in lysosomes. Following these studies, they suggested that the caveosome corresponds to the late endosomes/lysosomes and that the term should no longer be used. The interaction of the caveolar endocytotic carriers with the early endosomes occurs in a brief kiss and run fashion that preserves the caveolar structure [66].
2.2.1. Tools to study caveolae-mediated endocytosis
2.2.1.1. Chemical inhibitors
Polyene antibiotics such as filipin and nystatin, which sequester cholesterol from membrane structures [67], have been used to study both CME and caveolar endocytosis, which are both cholesterol-dependent. Methyl-β-cyclodextrin which selectively extracts cholesterol from the plasma membrane, thus inhibiting the invagination of the plasma membrane, is also used for inhibiting caveolae-mediated endocytosis [11]. However, these inhibitors can affect multiple endocytosis mechanism simultaneously, thus precautions should be taken when interpreting the results. These approaches are summarized in Table 1.
2.2.1.2. Genetic Tools
Modification of caveolin family proteins:
The caveolin family proteins seem to play the most important roles in caveolae-dependent endocytosis across different cells, making them an obvious target for manipulation of this pathway. The caveolin gene family has three members in vertebrates: caveolin-1, caveolin-2, and caveolin-3. Caveolin-1 exists ubiquitously in almost all kinds of tissue, although differences in its expression level occur across different tissues. Caveolin-2 is colocalized and co-expressed with caveolin-1, and requires caveolin-1 for structural stability and proper membrane targeting. Caveolin-3 has greater homology to caveolin-1 than caveolin-2, but it is expressed primarily in muscle cells [68]. Surprisingly, RNAi knockdown of caveolin-1 in COS-7 cells did not affect uptake of the cholera toxin B subunit, which is commonly assumed to be internalized by caveolae-mediated endocytosis [69]. However, cholera toxin B uptake and cholera toxin toxicity also occur in cells such as lymphocytes which lack well-defined caveolae and caveolins [70], suggesting that studies utilizing cholera toxin B to probe caveolae-dependent endocytosis may not be definitive. Interestingly, in caveolin-1 knockout mice, there was a complete absence of caveolar structures in the endothelial and epithelial cells of the lung, diaphragm, kidney and heart. Despite the complete depletion of caveolar structures in endothelial cells, transport of albumin and cholesterol, which are thought to rely upon caveolar function, appeared to be unaffected in caveolin-1 knockout mice compared with wild type mice. In addition, the abundance of proteins in lipid rafts, the lipid composition of lipid rafts, and the abundance of glycosyl phosphatidylinositol–anchored proteins normally enriched in lipid rafts from caveolin-1 knockout mice showed minimal differences compared with these measures in wild type mice [71]. Interestingly, another group reported reduced endocytosis of albumin by lung endothelial cells and aortic segment in caveolin-1 knockout mice compared with wild type mice [72]. Based on these studies, it appears that loss of caveolin-1 can efficiently disrupt the formation of caveolae, but this may not lead to effects on the endocytosis of caveolae-dependent cargoes. In caveolin-1 knockout mice, caveolin-2 levels were also dramatically reduced due to its degradation via proteasomes, suggesting that caveolin-1 expression was required to stabilize the caveolin-2 protein product [73]. In contrast, in caveolin-2 knockout mice, caveolae could still form and caveolin-1 maintained its localization in plasma membrane caveolae [74]. Caveolin-3 knockout mice showed an absence of sarcolemmal caveolae in skeletal muscle fibers, while caveolae were still present in endothelial cells from caveolin-3 knockout mice, suggesting that caveolin-1 expression is not affected by caveolin-3 knockout [75].
Modification of Cavins:
Cavins are essential structural components that reside on the cytoplasmic face of caveolae. There are four cavins in mammals. Cavins-1, −2 and −3 have a broad expression profile while Cavin-4 is muscle-specific. Cavin-1 knockout mice lack morphologically detectable caveolae in lung epithelium, intestinal smooth muscle or endothelial cells from all of these tissues, and also show markedly diminished protein expression of all three caveolin isoforms [76]. Deletion of Cavin-2 causes loss of endothelial caveolae in lung and adipose tissue, but had no effect on the abundance of endothelial caveolae in the heart and other tissues. Deletion of Cavin-3 does not interrupt caveolae formation in endothelial cells from most tissue [77]. Cavin-4 knockout mice showed normal caveolae formation and normal caveolin-3 localization at the plasma membrane in cardiomyocytes, indicating that Cavin-4 is not essential for that process [78].
2.2.2. Caveolae-mediated endocytosis in drug delivery
In endothelial cells, caveolae are thought to mediate transendothelial transport of certain macromolecules including albumin and lipids. Since the endothelium is one of the major barriers to drug transport from the circulation to tissue sites of drug action, caveolae transcytosis has been of great interest in the drug delivery field. However, this proposed process has long been debated and disputed in what has been called the “Vesicle Controversy” which questioned whether transendothelial transport occurred by vesicle-mediated versus paracellular mechanisms [79]. Due to a lack of specific tools to probe caveolae-mediated trafficking, many features of this pathway are still unclear. Several early studies suggested that caveolar endocytic carriers could directly transverse endothelial cells, bypassing the endosomal system [80–83]. Albumin was identified as one of the major cargos transported by this system. Utilizing biochemical approaches, the Schnitzer group identified several proteins including SPARC, gp60 (albondin), gp30, and gp18 which were located in the plasma membrane of cultured endothelial cells and that served as putative receptors for albumin. Furthermore, blockage of gp60 with antibodies prevented binding of albumin to the plasma membrane and reduced its internalization [82]. Minshall et al. further investigated this pathway, demonstrating physical proximity between gp60 and caveolin-1 [84]. Several early studies performed in cultured cells found caveolae-mediated endocytosis to be slower than CME [64, 85, 86], while others suggested caveolae were anchored to the actin cytoskeleton [87]. However, in one of the first in vivo studies of caveolar trafficking in lung tissue utilizing antibody-based targeting probes directed to aminopeptidase P enriched within caveolae, Oh et al. demonstrated endocytosis and accumulation of the probe in the perivascular space occurring as quickly as 5 s after intravenous administration [88]. Furthermore, using caveolin-1 knockout mice they also showed that endocytosis was caveolae-dependent for this particular probe. Other studies have revealed an increased transendothelial transport of albumin in caveolin-1 deficient mice, suggesting that albumin may also be transported through endothelial cells via paracellular or other routes [89].
Most studies regarding the dynamics, function and trafficking patterns of caveolae and caveolae-targeted drug carriers have been performed in cultured cells. As illustrated above, caveolar function may vary tremendously in in vitro models versus in vivo systems, For instance, there is a stark difference in the rates of caveolar endocytosis found in vitro (slower than CME) and in vivo (very rapid in endothelium) as discussed above [64, 85, 86, 88]. Furthermore, many of the tools used in the early studies of caveolae transcytosis are now known to lack specificity. For example, N-ethylmaleimide, commonly used as a caveolar transcytosis inhibitor [81, 83] was later demonstrated to elicit toxic endothelial damage and was also incapable of inhibiting transcytosis at non-toxic concentrations [90–92]. Simian virus-40 which was first believed to enter host cells specifically by caveolae endocytosis followed by transcytosis was later shown to also be endocytosed through a clathrin- and caveolae-independent pathway [93].
Caveolae cargo receptors such as gp60 and aminopeptidase P have been major targets to facilitate drug delivery via enhanced caveolae-mediated cellular uptake and, potentially, transcytosis [94, 95]. Early work on caveolae transcytosis used 20 nm gold nanoparticles (Au-NP) absorbed with albumin as a tool to study this process using electron microscopy. When placed on the luminal side of pulmonary microvessels, these particles bound to gp60 and induced caveolae-mediated endocytosis and transcytosis [90]. Another early study showed, with confocal microscopy imaging, that albumin-conjugated fluorescent polystyrene nanoparticles were internalized in a monolayer culture of bovine lung microvascular endothelial cells. Based on findings of a 70% co-localization of the internalized nanoparticles with caveolin-1, it was concluded that most of this uptake was due to caveolae endocytosis [96]. Since then, albumin has become a popular carrier or coat for a variety of nanomaterials, drugs and other biomolecules [94, 97–102]. The clinical potential of albumin-based targeting in cancer therapeutics was demonstrated by an albumin-bound, 130-nm particle form of paclitaxel (ABI-007) that demonstrated enhanced antitumor activity compared with equitoxic doses of Cremophor-based paclitaxel in five human tumor xenograft models [96]. ABI-007 was later FDA-approved and marketed as Abraxane®. Similarly, a polymeric doxorubicin conjugate nanoparticle decorated with albumin demonstrated robust transendothelial transport in HUVECs grown in Transwell plates [97]. Furthermore, these albumin-labeled nanoparticles showed improved antitumor efficacy in a human MCF-7 breast cancer xenograft model compared to conventional PEG-nanocarriers. The use of albumin in targeted drug carriers has been further reviewed elsewhere [103, 104]. Plasmalemma vesicle-associated protein (PV-1) is an endothelial-specific integral membrane glycoprotein associated with the stomatal diaphragms of caveolae, a specialized structure within the caveolae that are present only in endothelia of the continuous type including within lung and kidney; this protein has also received attention as a means of targeting uptake to these tissues via caveolae-mediated uptake [105–108].
2.3. Macropinocytosis
Macropinocytosis is an endocytotic process facilitating cellular uptake of extracellular fluid and soluble proteins, resulting in formation of cytoplasmic endocytotic vesicles with a diameter exceeding 0.2 μm termed macropinosomes [109]. This process occurs in a regulated manner through the activation of toll-like, chemokine or growth factor receptors, but can also occur constitutively in some cell types. Macropinocytosis is an actin-driven process. A ring of actin polymerization (circular ruffle) that may be up to several microns in diameter forms under the plasma membrane and either de novo or through folding of a linear ruffle, forms a cup-shaped invagination. Closure of the cup generates the macropinosome which enters the cell and matures.
The process of macropinocytosis is regulated by a series of small GTPases. Ras GTPases activate class-I phosphatidylinositol 3-kinases which generate membrane domains enriched in PIP3. These domains serve as docking sites for Rho GTPases that regulate the underlying actin remodeling and drive membrane ruffle formation. Once a macropinosome closes at the plasma membrane, the sequential steps of its maturation are coordinated by small Rab GTPases and phosphoinositides [110]. Rab5 and Rab34 have been shown to be involved in the early stages of macropinosome formation, facilitating their fusion with early endosomes [111]. At this stage of macropinosome maturation, a switch from Rab5 to Rab7 function facilitates its fusion with late endosomal/lysosomal compartments. Figure 1 illustrates schematically the process of macropinocytosis.
2.3.1. Tools to study Macropinocytosis
2.3.1.1. Chemical inhibitors
Inhibitors of macropinocytosis such as 5-(N-ethyl-N-isopropyl) amiloride (EIPA) [112], which prevents Na+/H+ exchanger thus lowering sub-plasma membraneous pH, and wortmannin, which leads to recession of membrane ruffles without forming macropinosomes [113], are widely used to study macropinocytosis. A screening study for macropinocytosis inhibitors from FDA-approved compounds showed that imipramine, phenoxybenzamine and vinblastine could potently inhibited macropinocytosis without exerting cytotoxic effects. Further mechanistic studies showed that imipramine inhibited membrane ruffle formation, a critical early step leading to initiation of macropinocytosis [114]. Stewart et al. showed that the diacylglycerol kinase inhibitor, R-59–022, could inhibit the internalization of fluorescently-labeled dextran which is internalized through macropinocytosis in Vero cells [115]. Furthermore, phellodendrine chloride, a plant-constituted alkaloid, inhibited macropinocytosis in pancreatic cell lines through inhibition of membrane ruffling [116]. These strategies are summarized in Table 1.
2.3.1.2. Genetic Tools
Myosins comprise a large superfamily of molecular motor proteins known to engage with actin filaments to drive vesicle motility, create propulsive force, and regulate actin remodeling. Particular myosins are involved in regulation of plasma membrane dynamics through interaction with actin filaments, a function important for maintaining mechanical force and membrane trafficking [117]. Myosin I family members are involved in regulating membrane tension and actin architecture, powering plasma membrane and organelle deformation and participating in membrane trafficking [118]. Myosin I single mutations in each individual isoform expressed in Dictyostelium discoidum did not show significant impairment of macropinocytosis, however, myosin Ia/Ib and myosin Ib/Ic double mutant strains of Dictyostelium discoidum exhibited a significantly slower rate of internalization of FITC-dextran compared with the wild type [119]. Knockdown of myosin Ic in HeLa cells caused a loss of lipid-raft-associated marker proteins from the cell membrane and significantly reduced macropinocytosis of fluorescent dextran [120]. SiRNA knockdown of the nonmuscle myosin II isoforms, -IIa and –IIb, in a neuroblastoma cell line resulted in opposite effects on macropinocytosis induced by phorbol myristate acetate or insulin-like growth factor. Myosin IIa knockdown significantly increased macropinocytosis, whereas myosin IIb knockdown significantly decreased macropinocytosis, with these changes correlated with upregulation and downregulation, respectively, of membrane ruffle formation [121]. Dendritic cells from myosin IIa knockout mice showed increased macropinosomes that were smaller and exhibited more static dynamics in parallel with an impaired ability to accumulate internalized antigens [122]. Recently, it was discovered that, beyond Ras [111], phosphoinositide 3-kinase [123], Src [124], and Rac [125] already implicated in stimulation of macropinocytosis, that microtubules, dynein motors, and the Arf6 effectors, the Jun kinase interacting proteins (JIP3/JIP4) scaffold proteins, are also required for macropinocytosis. This was determined in studies using siRNA knockdown of JIP3 and Arf6, which resulted in reduced macropinocytosis through inhibition of formation of macropinosomes [126].
2.3.2. Macropinocytosis in drug delivery
Lipid like materials, such as lipidoids [127], lipid nanoparticles [128], and lipopeptide nanoparticles [129] can successfully deliver siRNA into target cells through macropinocytosis. Polypeptide-derived nanoparticles such as histidylated polylysine polymers [130], pegylated poly-L-lysine [131], or elastin-like polypeptides [132] have demonstrated the ability to intracellularly deliver nucleic acid or drugs through macropinocytosis. Exosomes, extracellular vesicles carrying cargo such as non-coding RNA and proteins that are secreted from multivesicular bodies, have received considerable attention as natural drug carriers [133]. Macropinocytosis of exosomes has been demonstrated in epidermoid carcinoma and pancreatic carcinoma cell lines [134], in cerebral microvascular endothelial cell lines [135] and in neurons such as pheochromocytoma PC12 cells [136]. Though the precise mechanism of cellular uptake is controversial, some studies suggests that cell penetrating peptide-conjugated liposomes [137] or extracellular vesicles [138] can be internalized into target cells for intracellular delivery of biofunctional molecules through macropinocytosis. The studies utilizing colocalization with fluorescent dextran or inhibition of uptake with chemical inhibitors as tools to assess macropinocytosis, approaches which both lack specificity, may be viewed as less definitive [127, 129, 130, 132, 134], while a few studies have explored the genetic methods described in Section 2.3.1.2 to assess macropinocytosis with more specificity for the process [128].
2.4. Phagocytosis
Phagocytosis, defined as the cellular uptake of particulates (>0.5 μm) within a plasma-membrane envelope, is used by professional phagocytic cells such as monocytes, macrophages and dendritic cells to internalize microorganisms and present them to cells of the adaptive immune system. Nonprofessional phagocytes, such as fibroblasts, epithelial cells, and endothelial cells, can also perform phagocytosis to eliminate apoptotic cells. The first step in phagocytosis is the recognition of the particle by phagocytes. This recognition occurs via various receptors that recognize the particle as a target by directly recognizing specific molecular groups on the particle surface, e.g., nonopsonic receptors, or indirectly recognizing the opsonized particles through identification of host-derived opsonins bound to the surface of the particle, e.g., opsonic receptors. After recognition of the target particle, phagocytic receptors initiate signaling cascades that reorganize the plasma membrane and regulate the actin cytoskeleton to extend the cell membrane around the particle [139]. At the point of contact, a depression of the membrane called the phagocytic cup is formed. Then, the phagocytic cup closes at the distal end, forming a new phagosome. The phagosome undergoes a series of fusion and fission events with vesicles of the endocytic pathway, resulting in fusion with lysosomes to form a phagolysosome. During this process, there is a gradual decrease in pH and acquisition of digestive enzymes, leading to the digestion of the invader and recovery of antigens for presentation on the surface of the phagocyte [139]. This process is illustrated schematically in Figure 1.
2.4.1. Tools to study phagocytosis
2.4.1.1. Chemical inhibitors
The identification of phagocytosis inhibitors started decades ago. Cytochalasin B was shown to significantly inhibit the phagocytosis of S. aureus by human blood leukocytes and rabbit alveolar macrophages through inhibition of contractile microfilaments [140]. Adenosine, deoxyadenosine, and adenine arabinoside inhibited the phagocytosis of IgG-coated red blood cells (RBC) or zymosan by macrophages [141]. Mycotrienin, piericidin, and genistein were discovered as selective inhibitors of phagocytosis by macrophages through screening metabolites of Actinomyces [142]. Latrunculin A, an actin microfilament organization disruptor, also showed potent inhibition of phagocytosis of mouse peritoneal macrophages [143]. Thimerosal and p-nitrophenyl methyl disulfide could significantly inhibit Fc-receptor mediated phagocytosis of RBC by macrophages [144]. Other phagocytosis inhibitors also exist besides these mentioned. Since most phagocytosis inhibitors act through inhibition of the actin cytoskeleton, also implicated in other endocytosis mechanisms, this lack of specificity is a major concern when using these inhibitors for phagocytosis studies. General approaches are summarized in Table 1.
2.4.1.2. Genetic Tools
The particle recognition step has been widely targeted and is viewed as the most controllable step in manipulation of phagocytosis. Several molecules on the cancer cell surface such as CD47 [145], PD-L1 [146], beta-2 microglobulin subunit of the major histocompatibility class I complex) [147], and CD24 [148] can help cancer cells evade phagocytosis by macrophages through interaction with their respective receptors expressed on macrophages. Some bacteria can also evade phagocytosis by inhibiting opsonization [149–151], while others can directly disrupt cytoskeleton remodeling, inhibit plasma membrane extension or downregulate opsonic receptors, thus preventing engulfment by phagocytes [152, 153]. Some yeast can mask the antigenic protein by generating a thick polysaccharide barrier, thus escaping recognition by macrophages [154, 155].
Other than disrupting particle recognition, which has largely been probed from the perspective of evading phagocytosis, the molecular machinery for internalization can also be targeted for manipulating phagocytosis. A recent magnet-based phenotyping screening in a human myeloid cell line with phagocytic activity, U937 cells, identified genes that promoted or inhibited phagocytosis. Among the identified genes were components of the actin polymerization cascade known to be essential for phagocytic cup formation and including RAC1, DOCK2, members of the SCAR/WAVE complex and members of the ARP2/3 complex; the mTOR-associated regulator complex; lipid metabolism and sialic acid biosynthesis related genes; NHLRC2 which regulates RhoA-Rac1 signaling cascades controlling actin polymerization and filopodia formation; and the poorly characterized TM2D1, TM2D2 and TM2D3 genes [156]. Several substrate-selective phagocytosis regulators were also identified, such as the integrin related genes TLN1 and FERMT3, which facilitate the phagocytosis of zymogen and RBC, but had no effect on midbeads [156]. These discoveries have advanced the understanding of the molecular machinery of phagocytosis, and also identify specific proteins for potentially manipulating phagocytosis.
2.4.2. Phagocytosis in drug delivery
Phagocytosis is also an important mechanism for internalization of some DDS. Non-living bacterial envelopes (bacterial ghosts) which maintain cellular morphology and surface antigens, have been used to deliver DNA into antigen presenting cells through phagocytosis and consequently to induce immune responses in preclinical animal models, serving as potential vehicles for DNA vaccines [157–161]. Yeast-derived carriers have also been shown to successfully deliver siRNA into phagocytes through phagocytosis as potential candidate carriers for phagocyte-targeted gene therapy [162, 163]. Besides natural pathogen-derived delivery systems, other biological materials have been utilized for phagocyte- targeted delivery such as RBCs. Artificially-modified RBCs can be opsonized, then recognized and phagocytosed by macrophages, which makes them ideal phagocyte-targeted carriers [164]. RBC-mediated delivery of antiviral drugs into macrophages through phagocytosis showed significant inhibition of viral replication [165]. Dexamethasone, delivered by the same RBC-mediated system into macrophages, showed inhibition of NF-kB activation and TNF-α production [166]. Dexamethasone-loaded RBC therapy was advanced to clinical studies in chronic obstructive pulmonary disease patients, demonstrating a prolonged release of dexamethasone into plasma for up to seven days [167] and in Crohn’s disease, showing good tolerance and ability to maintain long term disease remission of the disease [168]. Consequently, dexamethasone-loaded RBC, patented as EryDex, showed significant improvement in neurological symptoms in patients with ataxia telangiectasia in a Phase II clinical trial [169]. Another RBC-mediated delivery system that active in clinical trials is L-asparaginase encapsulated in RBC, showing promising results in treatment of acute lymphoblastic leukemia in a Phase II clinical trial [170], acute lymphoblastic leukemia in a Phase II clinical trial [171] and pancreatic adenocarcinoma in a Phase II clinical trial [172]. Additionally, RBC-mediated drug delivery has also been studied in preclinical studies for treatment of lysosomal storage diseases (LSD), sickle cell disease [173] and hyperammonemia [174].
Besides the biologically-derived pathogen and RBC delivery systems mentioned above, ligand targeting can be utilized for phagocyte-targeting. Antigen-containing liposomes conjugated with IgG were internalized into dendritic cells, triggering immune responses in mice [175]. Anti-S. aureus antibody-conjugated antibiotics demonstrated intracellular killing of methicillin-resistant Staphylococcus aureus after opsonizing the bacterium and consequent phagocytosis into macrophages [176]. A doxorubicin loaded bionanocapsule displaying both Fc-binding domain and Fab-binding domains on its exterior could aggregate after mixing with IgG2a, followed by phagocytosis into macrophages, thus specifically delivering this drug to macrophages. This process was inhibited by latrunculin B, a non-selective phagocytosis specific inhibitor [177]. Notably, phagocytosis can also occur in non-professional phagocytes, such as epithelial cells. For instance, various vehicles have been developed to deliver drugs to retinal pigmented epithelial cells which can demonstrate the release of encapsulated dye intracellularly after phagocytosis into the cells [178–180].
Interestingly, macrophages can phagocytose and secrete oligonucleotides and proteins into the surrounding environment, which also makes them a delivery vehicle in addition to a target for delivery. It has been shown that macrophages can horizontally transfer proteins and nucleic acid such as DNA and siRNA into ischemic muscle [181], cancer cells [182] and neurons [183] in vitro and in vivo. Macrophage-derived extracellular vesicles are also used as drug carriers for chemotherapeutics due to their cancer-targeting ability [184, 185], and for protein delivery to the brain due to their ability to the cross blood brain barrier [135, 186].
Since a large fraction of administered nanoparticles may be sequestered and cleared by the reticuloendothelial system (RES), phagocytes can also be a hurdle for DDS. Polyethylene glycol (PEG) modification by PEGylation may provide a stealth functionality for DDS, as its addition can block serum protein binding to the surface of DDS and inhibit their phagocytosis [187]. CD47, an integrin-associated protein, is expressed on red blood cell and other cells as a “marker of self” [188, 189]. CD47 prevents RES uptake through interaction with SIRP-α on macrophages which is termed as “do-not-eat-me” signaling [190]. DDS coated with CD47-derived peptide significantly delayed macrophage-mediated clearance of nanoparticles and enhanced their persistent circulation [191, 192]. In recent years, interactions between CD47 on cancer cells and SIRPα on macrophages has been viewed as a potential immune checkpoint for cancer immunotherapy. Blockage of this immune checkpoint is currently in preclinical and clinical development for a variety of malignancies [193].
2.5. Other non-canonical endocytosis pathways
Although less mechanistic information is available regarding the role of molecular effectors, and selective strategies (both chemical and genetic) are lacking for their specific study, several additional endocytosis pathways offer special opportunities for drug delivery to discrete tissues/cells such as endothelium and T-cells. These processes are illustrated for the receptors of interest schematically in Figure 1.
CAM-mediated endocytosis
Cellular adhesion molecules (CAMs), such as intercellular adhesion molecule-1 (ICAM-1 or CD54) and platelet endothelial cell adhesion molecule-1 (PECAM-1) have each been exploited for intracellular delivery of biological materials. Multimeric anti-ICAM-1 or anti-PECAM-1 conjugates containing multiple antibodies can be internalized though CME-independent and caveolae-independent endocytic pathways into endothelial cells, a process termed CAM-mediated endocytosis [194]. CAM-mediated endocytosis plays an important role in endothelial targeting, since many CAMs are constitutively expressed on endothelium or can be induced by pathological conditions [195]. Monomeric anti-ICAM-1 can be similarly endocytosed; however the intracellular trafficking route is distinct between multimeric and monomeric ligands. Most of the multimeric anti-ICAM-1 conjugates become colocalized with perinuclear lysosomes, followed by degradation. In contrast, most of the monomeric anti-ICAM-1 conjugates become colocalized with a membrane compartment enriched in Rab11, and are ultimately recycled back to the cell surface with minimal degradation [196]. Such a difference offers the potential for different applications for each type of anti-CAM conjugates. A multimeric anti-ICAM-1 delivery system has been used to deliver the lysosomal enzymes as part of enzyme replacement therapy for LSD [197–201], for instance, while monomeric delivery vehicles may allow sustained intracellular delivery without degradation of the carrier.
The precise mechanism of CAM-mediated endocytosis is still not well-understood. This process is not inhibited by traditional CME or caveolae-mediated endocytosis inhibitors. It is, however, susceptible to amiloride, protein kinase C inhibitors, expression of the dominant negative dynamin 2 mutant, actin depolymerization, and Src kinase and Rho kinase inhibitors [194]. Na+/H+ exchanger family proteins, especially NHE1 and NHE6, may also play a role in distinct phases of CAM-mediated endocytosis and subsequent intracellular trafficking [202]. These approaches are summarized in Table 1.
Interestingly, the efficiencies of internalization of the same drug mediated by different pathways has been compared in the disease state utilizing CAM-mediated endocytosis and caveolae-mediated endocytosis. Endothelial proinflammatory activation, elicited by pro-inflammatory cytokines or lipopolysaccharides, have been shown to lead to the upregulation of CAMs, vascular cell adhesion molecule-1 (VCAM-1) in particular [203]. An important mediator of this process has been shown to be reactive oxygen species [204]. Shuvaev and colleagues pioneered the concept of using a targeted DDS to deliver the antioxidant enzyme, superoxide dismutase (SOD), specifically to endothelial cells affected by ischemia, inflammation, stroke and many other conditions [203]. SOD was conjugated with antibodies to PECAM-1 for targeted delivery to endothelium and was internalized via CAM-mediated endocytosis. Pro-inflammatory activation, as measured by downstream VCAM expression, was significantly downregulated both in vitro and in vivo by anti-PECAM-SOD, but not by its untargeted counterpart, polyethylene glycol (PEG)-SOD. Inspired by the great abundance of caveolae in endothelial cells, additional investigations using antibody-SOD conjugates highlight the relative potential of caveolae-mediated endocytosis relative to CAM-mediated endocytosis in this tissue for delivery of SOD [205]. SOD was fused to antibody to the caveolar cargo protein, PV-1, for targeted delivery to endothelial caveolae. Although anti-PV-1-SOD and anti-PECAM-SOD had similar tissue accumulation in vivo, only the former inhibited the pro-inflammatory cytokine accumulation in vivo during lipopolysaccharide-induced pulmonary inflammation.
IL-2R mediated endocytosis
Interleukin-2 receptor (IL-2R) mediated endocytosis is another example of a non-canonical endocytosis pathway of relevance to drug delivery that proceeds independently of known pathways such as CME and caveolae-mediated endocytosis. IL-2R normally functions in immune cells to bind interleukin 2 (IL-2) and transduce its signal intracellularly, with endocytosis comprising part of the signaling process. IL-2-functionalized nanocapsules or immunoliposomes can specifically target human and murine T cells in vitro and in vivo and be internalized through IL-2R-mediated endocytosis [206, 207]. IL-2 conjugated immunoliposomes delivered immunosuppressive reagents such as methotrexate into murine and human T cells following binding to and endocytosis of surface IL-2R [208]. Recently, this endocytotic route was defined as fast endophilin-mediated endocytosis (FEME), where endophilins cooperate with dynamin and the actin cytoskeleton in scission of vesicles. Inhibition of IL-2R endocytosis occurs via inhibition of dynamin, Rac, phosphatidylinositol-3-OH kinase, PAK1 and actin polymerization, and is activated by Cdc42 inhibition [209, 210] (Table 1). This same pathway has been implicated in ligand-mediated internalization of several G-protein-coupled receptors such as α2a- and β1-adrenergic, dopaminergic D3 and D4 receptors and muscarinic acetylcholine receptor 4, and the receptor tyrosine kinases EGFR, HGFR and VEGFR [209].
3. In Vivo Visualization of Endocytosis
Although visualization of endocytosis in cells has been enabled by many advances in light microscopy applications and the availability of diverse fluorescent probes, in vivo visualization of these processes continues to be more challenging due to the complex nature of multicellular organisms as well as a lack of specific and targeted probes. Here, we summarize recent progress on in vivo visualization of endocytosis in multicellular organisms (Figure 3).
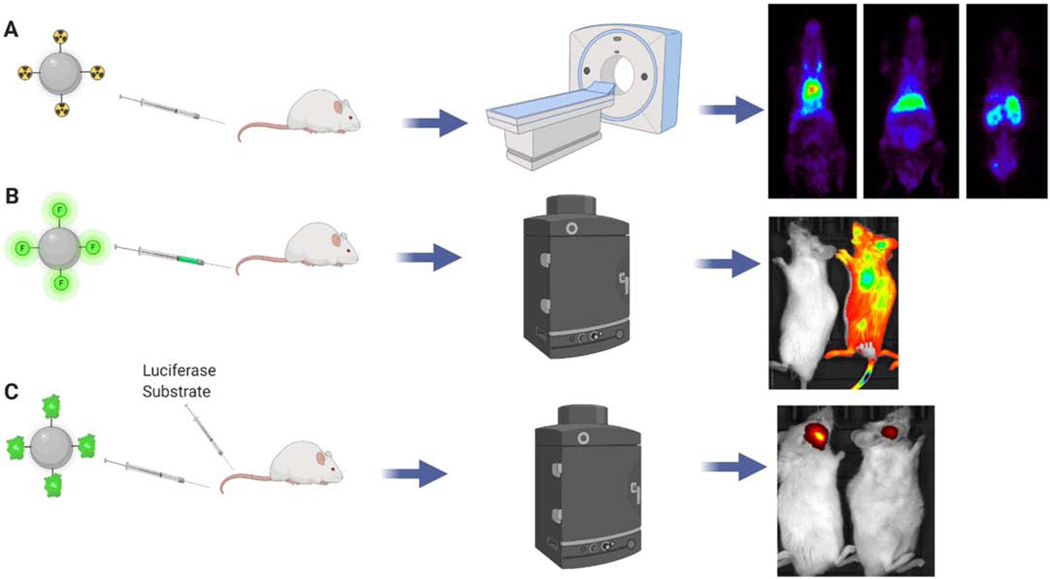
Figure 3. In Vivo tracking methods for DDS.
A) DDS labeled with radioisotopes can be tracked by PET scanning. B) Fluorescence labeling of the DDS can be used to track in vivo distribution by IVIS. C) The luciferase reporter system can also be used to track DDS distribution by IVIS after treating the animals with luciferase substrate. This schematic was created using BioRender.com
Radioisotope labeling has been widely used for tracking the in vivo distribution of drug carriers in animal models. Radioisotope iodine-124 labeled nanoparticle conjugated with anti-PECAM-1 antibody or anti- thrombomodulin antibody showed about 20-fold higher accumulation in the lung when compared with blood when analyzed by PET scanning [211, 212]. Copper-64 labeled nanoparticles conjugated with anti-ICAM-1 antibody showed significantly higher uptake in the lung when compared with control IgG [213]. Indium-111 labeled liposomes conjugated to anti-VCAM antibody showed significantly higher targeting to inflamed brain in mice when analyzed by single-photon emission computed tomography imaging (SPECT) [214]. I-125 labeled deformable nanogels conjugated with anti-PV-1, a marker found in lung caveolae, achieved significant targeting effects to the lung [215]. However, due to the limited resolution of this technique, it was hard to track endocytotic dynamics or single cell level details in real-time.
Fluorescence labeling and tracking is also widely used in small animals due to its fast acquisition and image processing times, thus allowing for high-throughput imaging in real-time. Near-infrared fluorophores are more commonly used than fluorophores emitting energy in the visible range due to better tissue penetration, less absorption and scattering of emitted photons and less tissue autofluorescence in this range [216]. However, this technique does not provide enough sensitivity for imaging of cellular and molecular processes in intact living tissues with high resolution.
Several luciferase reporter systems have been developed to track DDS dynamics in vitro and in vivo [217–221]. Biodistribution of exogenously administered exosomes derived from luciferase reporter gene transfected parental cells were visualized in vivo with an in vivo imaging system (IVIS) after the animal was treated systemically with luciferase substrate [217, 219, 220]. Lipid nanoparticles carrying mRNA encoding the luciferase reporter gene was also used to evaluating successful delivery of mRNA into target tissues when given by different route by IVIS after systemic administration of D-luciferin [222, 223]. One advantage of using the luciferase reporter is its high signal-to-noise ratio, in contrast to fluorescence imaging which can be affected by tissue autofluorescence. Another advantage is that the luciferase reporter has a low risk of photobleaching and phototoxicity which is always a concern for fluorescence imaging. A drawback of using luciferase is that compared with fluorescent signals, the signal of a luciferase reporter is relatively dim because it is dependent on the amount of local substrate. The toxicity of the substrate, its bioavailability, and the half-life of luciferase should also be taken into consideration when applying it for in vivo imaging.
Intravital Microscopy (IVM) encompasses a series of light microscopy-based techniques that can be used to image several biological processes in live animals. These techniques are useful tools to study endocytosis processes in vivo [224] especially when combined with two photon microscopy which ensures deeper tissue penetration of the light source. IVM combined with indirect immunofluorescence has been used to examine how a specific regulatory protein of endocytosis affected maintenance and formation of the intercellular canaliculi in the acinar cells of the submandibular salivary glands in live mice [225]. IVM has also been used to visualize different endocytosis pathways (CME or fluid phase) as well as the intracellular trafficking of fluorescently labeled ligands in different segments of kidney tubules [226], intestinal epithelium [227], liver [228] and tumors in situ [229, 230]. The effects of nanoparticle size, shape, charge and material composition on uptake and membrane trafficking in live animals has also been studied by IVM [231]. A significant advantage of IVM is that it allows the study of endocytosis processes in different cell types at the same time in a physiological environment. With the help of different surgical and injection techniques, IVM has also enabled the study of endocytosis in polarized epithelia in salivary gland. Basolateral endocytosis was followed by delivery of fluorescent probes from the vasculature [232], whereas apical endocytosis was studied by retro-diffusing a fluorescent probe through secretory ducts [233]. Methods have also been developed to visualize the dynamics of extracellular vesicle uptake and translation of extracellular vesicle derived cargo mRNA in cancer cells in vivo in tumor-implanted mice [234].
Super-resolution techniques which provide superior spatial and temporal resolution have also advanced the in vivo visualization of endocytosis to a great extent. A recently developed pulse–chase technique for subdiffractional tracking of internalized molecules (sdTIM) was able to visualize the endocytosis of fluorescently tagged molecules and their presence in individual signaling endosomes and SVs in presynapses, which provided a great tool to understand endocytic pathway dynamics in vivo [235]. A combination of super-resolution microscopy and single-molecule data analysis, i.e., two-color Stochastic Optical Reconstruction Microscopy (STORM), addressed the size and positioning of nanoparticles inside cells and probed their interaction with the cellular machineries at nanoscale resolution, which may potentially be applicable to in vivo studies [236].
Interestingly, Masedunskas et al. have demonstrated that the dynamics of internalization of Tf and dextran, two molecules that traffic via distinct endocytosis mechanisms, are substantially altered in ex vivo cultured salivary gland stromal cells when compared with in vivo studies in the live animal, which indicates that the surrounding environment exerts effects on endocytosis and membrane trafficking [237]. This intriguing finding illustrates that in vitro and ex vivo endocytosis processes may not recapitulate physiological processes in a live organism. Whenever feasible, evidence from in vivo tests adds great strength in supporting a conclusion regarding in vivo mechanisms of action.
4. Endocytosis and DDS physical properties
4.1. Size of DDS
Considerable effort has been expended to understand how the physical properties of nanoparticles and microparticles, including size, deformability, and shape, impact endocytotic uptake. Interpretation of such studies has not always been straightforward for many reasons including the predominant use of non-specific chemical inhibitors to define the pathways under study, the use of different cell models which manifest differential expression of the endocytic pathways of interest, and the lack of studies in vivo which more accurately recapitulate pathways such as caveolae-mediated processes in endothelium. For instance, to understand the size constraints of CME, Jiang et al. screened Herceptin-coated Au-NPs within a 2–100 nm size range and suggested the optimal size range for uptake via CME to be 40–50 nm [238]. This is consistent with another research study which demonstrated that 45 nm Au-NPs penetrated cells via CME. However, in a different study, CME was found to be able to accommodate 50–200 nm metal hydroxide nanoparticles [239].
Despite substantial overlap in capacities across the two pathways, the size constraints imposed by caveolae-mediated endocytosis appear more limiting than for CME. A series of folate-decorated nanoparticles with size ranging from 50 to 250 nm were fabricated to study their uptake in retinal pigmented epithelial cells. The internalization of 50 nm and 120 nm nanoparticles was found to be mediated by both CME and caveolae-mediated endocytosis. However, 250 nm nanoparticles were internalized only via caveolae-mediated endocytosis [240]. Additional studies have compared the effects of particle size on the preference for uptake via CME and caveolae-mediated endocytosis. Rejman et al. screened a series of fluorescent polystyrene latex beads with defined diameters ranging from 50–1000 nm in non-phagocytic B16 tumor cells. Particles up to 500 nm were internalized via an energy-dependent endocytosis process. The formation of clathrin-coated pits was observed when particles were smaller than 200 nm, suggesting the dominance of CME for particle internalization in this size range. As particle size increased, the authors suggested that caveolae-mediated endocytosis became predominant, supported by their findings that cholesterol depletion (a non-specific strategy for inhibition of caveolae-mediated endocytosis) began to exert a stronger inhibitory effect on particle internalization [241]. Despite the apparent ability to internalize larger particles, smaller nanoparticles are still internalized by caveolae-mediated endocytosis at a much higher rate than larger nanoparticles. Wang et al. observed that internalization of albumin-coated polystyrene nanoparticles by bovine lung microvascular endothelial cells targeted to caveolae was dependent on nanoparticle size and time of exposure. Over the same time period, internalization of smaller nanoparticles (20 and 40 nm) was 5–10 times greater than that of larger (100 nm) nanoparticles [96].
Deformable nanocarriers have also been utilized to overcome the presumed size limitations of caveolae. Lysozyme-dextran nanogels of ~150nm and ~300nm mean diameter were able to target the PV-1 cargo protein in caveolae of lung endothelial cells while rigid polystyrene particles of the same sizes could not. This in vitro data suggested that caveolae-mediated endocytosis is capable of mediating the cellular uptake of particles significantly larger than the ~50 nm diameter of the entry aperture imposed by plasma membrane invagination [242]. This size limitation was reinforced by Ho et al. in a study probing the effects of the specific proteins constituting the nanoparticle corona on cellular uptake and transcellular permeability of polystyrene nanoparticles across HUVECs [243]. By varying the biological identity of the proteins constituting the nanoparticle corona as well as the particle size, these investigators showed that uptake by caveolae-mediated endocytosis was more size-dependent than surface-dependent, with smaller nanoparticles shown to be more caveolae-dependent for cellular entry than larger nanoparticles. However, the cellular uptake of nanoparticles did not fully recapitulate their transcellular permeability, as higher uptake by HUVECs did not necessarily translate to a higher transcytosis rate, indicating that targeting of caveolae alone is not enough to induce transendothelial transport of drug carriers. Future studies should benefit from new genetic tools that may more accurately modulate the specific endocytotic pathways under investigation.
Particles bigger than 1 μm are assumed to elicit phagocytic responses and to be internalized through phagocytosis in macrophages. Early studies showed that macrophages internalize high-molecular-weight poly(vinylpyrrolidone) much faster than its low-molecular-weight counterpart [244]. Koval et al. studied the size effects of IgG-opsonized polystyrene microbeads with defined sizes ranging from 0.2 to 3 μm on cellular uptake by mouse bone marrow-derived macrophages. Relative to smaller particles (0.2 to 0.75 μm in diameter), the internalization of larger particles (2 μm and 3 μm), were more likely to be internalized by phagocytosis as evidenced by the increasing dependence of internalization on actin polymerization. Smaller microbeads tended to undergo CME, supported by the fact that inhibition of CME with potassium depletion (a non-selective chemical strategy) downregulated the uptake of smaller beads more effectively [245]. The fact that HeLa cells were able to internalize particles of 1 μm, 2 μm and even 3 μm in diameter suggests either that non-phagocytotic cells can be induced to perform phagocytosis, as mentioned earlier [246], or that this uptake is occurring via actin-dependent CME or caveolae-mediated pathways as suggested by the authors.
CAM-mediated endocytosis, occurring through non-canonical endocytosis, has also demonstrated a size preference. Since CAM-mediated endocytosis is predominantly reported in endothelial cells, a series of anti-PECAM antibodies decorating streptavidin-beta galactosidase to form conjugates with diameters ranging from 80 nm to 5 μm were tested on HUVECs to study whether their cellular uptake was size-dependent. Only anti-PECAM conjugates smaller than 350 nm were successfully internalized [247]. In another study, anti-PECAM and anti-ICAM antibodies were used as targeting moieties to direct the antioxidant enzyme, SOD, to the endothelium while under oxidative stress. By varying the molar ratio of anti-PECAM antibodies or anti-ICAM antibodies to SOD, a series of antibody-SOD conjugates with different sizes ranging from approximately 50 nm to 300 nm were synthesized to study the size effects on the internalization process. A clear negative correlation between internalization and particle size was observed, showing that smaller particles had a higher internalization efficiency [248]. Similar to caveolae-mediated endocytosis, elasticity of nanoparticles also elicited a strong effect on CAM-mediated endocytosis. Anselmo et al. synthesized a series of anti-ICAM antibody-coated polyethylene glycol (PEG)-based hydrogel nanoparticles with uniform size of 200 nm but of differing rigidity (i.e., elastic modulus). The hard nanoparticles exhibited higher cellular uptake by bEnd.3 endothelial cells relative to their soft counterparts [249].
4.2. Shape of DDS
Shape is another key parameter influencing cellular uptake. Its effects on phagocytosis have been intensively studied by Champion and colleagues [250]. Their initial studies explored polystyrene particle phagocytosis by alveolar macrophages, varying particle size and shape, and concluding that the initiation of phagocytosis was dependent on the local particle shape that first comes into contact with the macrophage. This local particle shape was measured by the tangent angles Ω, defined as the angle between the membrane at the point of initial contact and the mean direction of the tangent drawn to the target contour from the point of initial contact. Phagocytosis was initiated only when a high length curvature region (Ω<45°) came into contact with the membrane. Contact with a low length curvature region r (Ω>45°) resulted in simple membrane spreading but not internalization. Particle size, on the other hand, dictated whether phagocytosis could be completed, given the possibility that the particle size may exceed the cell volume [251]. Therefore, minimizing the higher length curvature region has the potential to help nanocarriers to elude phagocytosis. This proposal was validated in a subsequent in vitro study, where highly stretched worm-like polystyrene particles, characterized by their very high aspect ratio (>20), exhibited minimal phagocytosis in rat alveolar macrophage cell lines [250].
Shape effects on CAM-mediated endocytosis have also been reported. The shape preference for CAM-endocytosis was ranked as rod-like > spherical > polymorphous [248, 252], consistent with the shape preference of endothelium to adhere ICAM-antibody-coated nanoparticles of different shapes. This ranking is subject to constant modification as more studies are conducted. The internalization of rod-like versus spherical ICAM-antibody-coated particles into a rat brain endothelial cell line was tested under both static culture condition and flow conditions. During the 30 min incubation, ICAM-antibody-coated nanorods were taken up more efficiently than ICAM-antibody-coated spheres under flow conditions. Nonspecific IgG-coated nanoparticles, did not demonstrate any notable differences in cellular uptake between two shapes under the same conditions [252]. Shuvaev et al. demonstrated that anti-ICAM antibody-coated spheres were more rapidly internalized into HUVECs than were polymorphous anti-ICAM antibody conjugates [248]. Non-phagocytic cells, such as HeLa cells, also exhibited a preference for rod-like hydrogel nanoparticles with a higher aspect ratio, which are internalized ~4X faster than cylindrical 200 nm nanoparticles with an aspect ratio=1 [246]. Shimoni et al. further reported that spherical hydrogel capsules were taken up faster by HeLa cells than their rod-shaped counterparts [253]. The internalization shape preference in HeLa cells can be therefore be approximated as sphere > rod-like> symmetrical cylinder. This shape preference has been supported by other studies and observed in other cell types as well. Tf-coated Au-NPs have been shown to be internalized via CME. Spherical-shaped Au-NPs exhibited faster cellular uptake relative to their rod-shaped counterparts[254]. Spherical particles showed higher uptake relative to cubic particles in SK-BR-3 cells, a non-phagocytic cell [255]. Particle shape may also play a role in regulating the choice of endocytotic entry pathway. By studying the shape effects of PEGylated mesoporous silica nanoparticles on cellular uptake pathway in HeLa cells, Hao et al. suggest that spherical particles favor CME for internalization, while particles with larger aspect ratio are more likely to be internalized via caveolae-mediated endocytosis [256]. Linear and branched poly(ethylenimine)s (PEIs), which are used as polymer therapeutics and vectors for cytosolic delivery also showed differential endocytosis efficiency and mechanisms in murine melanoma cells. Branched PEI showed significantly higher extracellular binding and uptake than linear PEI. Branched PEI were predominately internalized using cholesterol-dependent pathways, whereas internalization of linear PEI appeared to be independent of clathrin and cholesterol [257].
4.3. Other Properties
The kinetics of cellular uptake is also influenced by the surface charge of the DDS. Positively-charged nanoparticles are generally more rapidly and effectively internalized than their negatively-charged counterparts, possibly due to charge-charge interactions with the plasma membrane [246]. However, few studies have explored the combined influence of shape, size and charge combined with the additional complexity of endocytotic targeting moieties such as TfR, monoclonal antibodies, or albumin, for instance. In such a situation, the factors that dominate to dictate internalization are not well understood. However it is clear that size, shape, rigidity, surface charge and targeting moiety valency of DDS influence their endocytosis, beyond the inclusion of specific targeting moieties [258].
5. Intracellular Trafficking and Targeting of DDS
All DDS entering cells through endocytosis are, at first, confined to intracellular vesicles, the identity of which varies depending on their mode of internalization. Vesicle contents can be recycled, transcytosed, or be passively or actively targeted into the endo-lysosomal pathway. Once reaching the endosomes, with assistance, DDS may also exit endosomes for access to cytosol and/or targeting to other intracellular compartments. To maximize desired therapeutic effects, the ability to transport some DDS to a specific cellular compartment or domain such that encapsulated or attached drug has maximal access to its target can be advantageous. However, default intracellular trafficking may result in sufficient drug bioavailability in many cases. For instance, targeting to lysosomes requires little additional engineering for many DDS that are internalized via endocytotic pathways that traffic to this compartment by default. Degradation of biodegradable DDS in the lysosomes can, in many instances, result in slow release of encapsulated drug into the cytosol. However, other therapies, including siRNA and gene delivery systems, may require assistance to exit the endo-lysosomal system to reach cytosol and/or nuclei to be maximally effective. Endo-lysosomal exit and subsequent mitochondrial targeting are likewise essential for therapies addressing mitochondrial dysfunction. The intracellular trafficking patterns of a specific DDS are, like its cellular uptake, dependent on its size, shape, and surface properties as well as any appended targeting motifs. For more detailed recent reviews of subcellular targeting approaches see [259–261]. Here we discuss a few representative studies which outline the benefits of targeting intracellular trafficking focusing on: recycling/exocytosis and transcytosis; lysosomal targeting; endosomal escape; and targeting to specific cellular organelles (Figure 4).
Figure 4. Schematic illustration of intracellular trafficking related to drug delivery.
After cellular uptake, DDS are usually transported to endosomes, which act as a sorting center for intracellular transport. DDS can be further transcytosed, exit endosomes (with assistance), or be passively or actively targeted to lysosomes. DDS that exit endosomes may be further targeted to other compartments such as mitochondria and ER. Lys: Lysosomes. This schematic was created with BioRender.com
5.1. Recycling/Exocytosis and Transcytosis of DDS
Endocytotic pathways engage in various extents of recycling traffic from early and late endosomes and lysosomes. DDS, once internalized, can be directed for secretion from cells at several points of the endo-lysosomal system. They may be exocytosed from recycling endosomes or accumulated in multivesicular bodies that may either be exocytosed or fused with lysosomes. DDS may also be exocytosed directly from the lysosomes [262]. Through electron microscopy analysis, Chithrani and Chan illustrated this process through visualization of Au-NPs, showing that Au-NPs about to be removed from cells were first localized in late endosomes and lysosomes, and then in vesicles apparently moving toward the cellular membrane where they eventually docked and released their contents [254]. The process of nanoparticle elimination, although crucial to the understanding of a particular DDS’s accumulated therapeutic dose and its ability to traverse barrier tissue, is far less well understood than its uptake mechanism, partly due to methodological limitations, as degradation and excretion of many DDS are difficult to distinguish experimentally [262]. Chu et al. demonstrated that although the uptake mechanism of silica nanoparticles was similar across several cell types, that their secretion patterns varied and thus the ultimate retention of the internalized particle [263]. Other studies have demonstrated varying exocytosis rates from the same cell type, dependent on the size and shape of Au-NPs [254]. In addition to physical parameters, the influences of targeting ligands can play a major role. For further reading, we refer to the following reviews [259, 264, 265].
For most DDS administered parentally or orally, their tissue targets lie beyond the intervening endothelial and epithelial barriers which must be crossed. To accomplish this, transcytotic trafficking pathways present in polarized cells can be targeted. As discussed in Section 2.2, caveolae-mediated endocytosis has been successfully targeted to facilitate transendothelial transport. To facilitate transepithelial transport, receptors such as the Fc and polymeric immunoglobulin receptors responsible for transport of immunoglobulins across the cells can be utilized. Pridgen et al. compared untargeted and FcRn-targeted nanoparticles administered orally, and found an enhanced absorption efficiency for the targeted nanoparticle in vivo and in vitro. As proof of principle, a therapeutic dose of insulin was delivered utilizing this system which elicited a prolonged hypoglycemic response in wild-type mice which was abolished in FcRn knockout mice [266]. pIgR targeting has also been utilized by Ferkol et al. to deliver a bifunctional fusion protein, comprised of a single-chain Fv directed against secretory component, the extracellular portion of the pIgR, fused to human α1-antitrypsin. This bifunctional protein was delivered across the respiratory epithelium in a cell monolayer as well as in a human tracheal xenograft model in a receptor-dependent manner [267, 268].
The blood-brain barrier constitutes a significant challenge to the delivery of drugs into the brain. Targeting of receptor-mediated transcytosis is one approach that has been taken to overcome this barrier. Several receptors capable of inducing receptor-mediated transcytosis are present in the blood-brain barrier, including the insulin receptor, TfR, and receptors responsible for lipoprotein transport. A plethora of both in vitro and in vivo studies targeting these receptors has been performed, often utilizing antibodies directed towards the receptor that are conjugated with a therapeutic component. For further reviews on this topic see [269–271].
Several less well-characterized mediators of transcytosis have also been suggested. As an example, a peptide encompassing the RGD targeting motif to αvβ3 or αvβ5 integrins called iRGD (CRGD[K/R]GP[D/E]C) has, in a pathway involved in integrin and neuropilin-1 (NRP-1) binding, been suggested to activate a transcytotic pathway capable of enhanced drug delivery to solid tumors [272]. Upon integrin binding of iRGD, the peptide was proteolytically cleaved to release the C-terminal RXXR/K sequence, (CendR) motif which interacted with neuropilin-1 to activate a transcytotic pathway which can enhance the delivery of common cancer therapeutics [252]. Hseuh et.al also demonstrated unconventional transcytosis of an ELP nanoparticle across the lacrimal gland acinar epithelium which may be utilized to mediate continuous delivery of encapsulated or attached drug to the ocular surface through release in tears [273]. With increasing availability of knockout mouse models enabling greater rigor for control studies and utility of in vivo imaging approaches, more mechanistic information on transcytosis of different DDS should be increasingly available.
5.2. Lysosomal Targeting
When internalized through endocytosis, without recycling, the contents of many internalized DDS continue to traffic through the endosomal pathway to the lysosomes by default (passive targeting), where contents are digested. Many DDS comprised of biodegradable polymers are intended to be passively effluxed to lysosomes, thus resulting in the slow release of drug to cytosol as DDS are slowly broken down [274]. Enzymatically-degradable polymers investigated as DDS include three main categories: proteins, polypeptides and polysaccharides [275]. Extracellular matrix protein polymers, such as collagen [276, 277] and elastin [278] have been used as DDS to deliver small molecule drugs, proteins and genes. Additionally, both natural polypeptides, such as Poly(γ-glutamic acid) [279], and synthetic polypeptides, such as Poly(L-glutamic acid) [280], Poly(aspartic acid) [281] and ELPs [282–285] have been investigated to deliver small molecules, peptides, proteins and genes/plasmids. Finally, some types of polysaccharides, represented by hyaluronic acid [286] and chitosan [287] are also ideal biodegradable polymers for lysosomal delivery.
Due to the accessibility to lysosomes for materials from multiple endocytotic pathways, endocytotic targeting and delivery to lysosomes has also been actively targeted facilitate the treatment of lysosomal storage diseases (LSD). LSD are characterized by aberrant accumulation of undegraded metabolites in lysosomes, and caused by compromised hydrolytic enzyme activity usually associated with a genetic deficiency in protein expression [288]. Enzyme replacement therapy is an established treatment strategy for LSD. When newly synthesized, many lysosomal enzymes display a mannose-6-phosphate (M6P) motif that confers capture in the trans-Golgi network and sorting to lysosomes. In some cases, endogenous lysosome enzymes that would normally have been directly sorted to lysosomes may be secreted into the extracellular environment. These enzymes also display M6P. Receptors to M6P are expressed on the cell surface of most cell types to salvage these secreted lysosomal enzymes by endocytosis and eventual trafficking to lysosomes. Many recombinant enzymes used for enzyme replacement therapy may not have the proper post-translational modifications required for M6P receptor recognition. In order to enable glycosylation-independent internalization of extracellular enzyme, LeBowitz and colleagues designed a chimeric protein by fusion of β-glucuronidase with a fragment of insulin-like growth factor II (IGF-II) which binds to a different domain of the M6P receptor. This IGF-II tagged enzyme was more effective than untagged enzyme in reversing lysosome storage deficiency of glycosaminoglycans [289]. Other receptors beside the M6P receptor which are internalized by CME may also be targeted for glycosylation-independent internalization of extracellular lysosomal enzymes. In another example exploiting the same chimeric protein concept, α-L-iduronidase or acid α-glucosidase were designed to display the receptor-associated protein to target LDLR. Fusion with the receptor-associatetd protein increased the cellular uptake of these recombinant enzymes to lysosomes by at least an order of magnitude [290].
Multivalent ICAM-1 targeted delivery is another strategy for lysosomal targeting. Nanoparticles conjugated with anti-ICAM-1 antibodies are internalized through a CME- and caveolae-independent endocytosis process termed CAM-mediated endocytosis, as discussed in Section 2.5. Following internalization, the nanoparticles go through sequential fusion with endosomes and lysosomes, and eventually are degraded in the lysosomes. Taking advantage of the natural intracellular trafficking pathway of ICAM-1 targeted nanocarriers, several strategies have been applied to deliver disease-related enzymes specifically to the lysosomes. ICAM-1-targeted carriers significantly facilitated the endocytosis and trafficking to lysosomes of acid sphingomyelinase, thus significantly increased enzyme activity in organs and reduced lung sphingomyelin storage and macrophage infiltration in a murine model of Type B Niemann-Pick Disease [291, 292]. This strategy also showed potential in delivery of active enzymes into lysosomes in model human neurons [293]. RGD-functionalized liposomes and polymers have also been used for lysosomal-targeted delivery of active enzymes in LSD models [294, 295], following the natural intracellular trafficking pathway of α5β1 intergrin [296]. Besides ICAM-1 and integrin, some other plasma membrane proteins such as LDLR, VEGFR, EGFR and VCAM-1 are naturally trafficked to lysosomes following endocytosis into the cell [297–300]. Targeting these molecules is also viable for achieving active lysosomal targeting.
5.3. Endosomal Escape
Because many drug targets exist in cytosol or other membrane compartments not directly accessible from the endo-lysosomal pathway, there has been interest in exploration of different endosomal escape mechanisms including: pore formation in the endosomal membrane; fusion with the endosomal membrane; endosomal membrane destabilization using the “proton sponge effect”; or protein conformational changes within the endosome which release drug for diffusion through the membrane [301, 302]. Many of these mechanisms are modeled on studies of pathogen entry and subsequent endosomal escape. For instance, to deliver their genome to the host cell cytoplasm, many viruses have developed sophisticated mechanisms to facilitate endosomal escape, with these motifs constituting a reservoir of potential escape agents. Several virus-derived proteins and peptides have been shown to be fusogenic or to drive endosomal pore formation in an acidification-dependent manner [303] such as the HA2 subunit of haemagglutinin protein [304–307], the diINF-7 peptide from influenza virus [308–310] and the gp41 transmembrane protein from human immunodeficiency virus [311, 312]. Similarly, some bacteria can also hijack intracellular trafficking pathways to promote their replication by secreting pore forming toxins, including listeriolysin O [313–316], diphtheria toxin [317, 318] and Pseudomonas aeruginosa exotoxin A [319–323]. Inspired by viral- or bacterial-derived agents, several synthetic peptides are known to have endosomolytic properties. Based on the N-terminal sequence of the HA2 subunit of influenza virus haemagglutinin, two amphipathic peptides, KALA and GALA, were constructed. Both of these peptides are pH-sensitive and undergo a structural conversion from a random coil to an α-helix at lower pH, destabilizing endosomal membranes and promoting cytosolic release of their contents [324–330]. Finally, in order to counteract the pH-buffering capacity of cationic polymers, such as polyethylenimine [331, 332] and polyamidoamine [333], and maintain the desired acidic endosomal pH, more protons and counter-ions are transported into the endosomes, leading to increased osmotic pressure and eventually causing endosomal swelling and rupture. This proton sponge effect can therefore also faciliate endosomal escape of therapeutics [334].
5.4. Targeting to Specific Cellular Organelles
5.4.1. Nucleus:
Gene therapy, the delivery of DNA or RNA to replace a non-functional gene or to suppress the expression of a harmful gene product, constitutes a powerful tool for the treatment, cure or prevention of many diseases. For optimal insertion and expression of many transgenes, delivery to the nucleus is desirable. One widely used strategy for nuclear targeting is the attachment of a nuclear localization signal (NLS) to the DDS [335]. NLS are short peptides that bind to cytoplasmic importins, proteins localized to the perinuclear region of the cell. After endosomolysis, cytoplasmic binding to importins may occur and NLS-conjugated DDS appear to enter the nucleus via active transport through the nuclear pore complex in cultured cells [336–339]. In vivo studies have also demonstrated that NLS-conjugated systems have significantly higher efficiency at delivery of DNA into the nucleus relative to nontargeted systems in a mouse model. Notably, the size of the drug carrier and density of the NLS targeting motifs can significantly impact the efficiency of trans-nuclear membrane transport [337].
A few studies have explored folate receptor (FR)-α, as another possible strategy for nuclear targeting due to a report that it is able to traffic to the nucleus [340]. FR-α targeted carriers were able to increase the accumulation of cargo in the nucleus relative to nontargeted carriers in cells [341, 342]. An extensive literature exists on the targeting of DDS to the FR-α [107–109] and many therapies have advanced to clinical trials including antibody–drug conjugates, folate-drug conjugates, radioimmunoconjugates and even FR-targeted CAR-T cells, based on findings that FR is highly upregulated in a many solid tumors [343–346]. However, most developments in FR targeting have been made based on observed enhancement of drug uptake through FR endocytosis rather than through FR targeting to the nucleus. Early initiatives in FR targeting were based on the premise that drug internalization and accumulation was via cavaeolae-mediated transport or “potocytosis”; although this concept was later questioned. It is now thought that FR-medated uptake occurs through multiple endocytotic routes [347].
5.4.2. Mitochondria:
Mitochondrial targeting can be achieved through several approaches, once DDS undergo endosomolysis. Conjugation of DDS with a peptide mitochondrial leader sequence that is directed to the mitochondrial protein import machinery (translocases) of the inner or outer membrane is the most common approach [348]. Another approach has utilized lipophilic molecules with a delocalized positive charge such as the triphenylphosphonium cation, which targets the negative charge of the mitochondrial matrix to cross the mitochondrial membranes [349]. Further, Yamada et al. have developed a liposomal based system, called the MITO-porter, that enters cells through macropinocytosis, exits the endolysosomal pathway and enables mitochondrial drug delivery through direct fusion with the mitochondrial membrane [350, 351]. These and other mitochondrial targeting approaches are further reviewed in [352, 353].
5.4.3. Endoplasmic Reticulum:
The ER, a critical cellular organelle which facilitates the proper folding and transport of transmembrane and luminal proteins, is another important organelle for DDS interventions. ER-targeted delivery methods have potential in treating cancer, diabetes, cardiovascular diseases and neurodegenerative diseases [354]. Several ER targeting moieties have been developed and tested in cultured cells; some approaches have been based on naturally occurring sorting motifs, such as the Lys–Asp–Glu–Leu (KDEL) peptide and the dilysine signal (LL), while others are based on chemical modification including addition of tosyl groups or 4,4′,4″,4′″-(porphyrin-5,10,15,20-tetrayl)tetrakis(N-(2-((4-methylphenyl)sulfonamido) ethyl) benzamide [355–358]. Since the loading of antigenic peptide onto Major Histocompatibility Complex I (MHC I) proteins also happens in the ER, studies have shown that attachment of an ER signaling sequence to antigenic peptides can facilitate their translocation to the ER and eventual presentation on the cell surface, thus enhancing their immunogenicity [359]. This ER-targeted strategy was successfully applied in a DNA vaccination approach which induced CD8+ T cells and conferred anti-tumor immunity in animal models [360, 361].
6. Conclusion and Future Perspectives
Despite their lack of specificity, chemical inhibitors are still the most commonly used tools to dissect endocytotic cargo entry mechanisms because they are easily available and simple to use. Limitations in their use include the fact that their efficiency of inhibition can be cell-type dependent and that they may affect more than one endocytotic pathway. Even the newer chemical inhibitors lack complete specificity for a specific endocytotic pathway. In addition, other cellular events such intracellular trafficking, exosome biogenesis, mitochondrial membrane potential, and cytoskeletal organization can be indirectly affected, introducing more confounders. Thus, caution should be applied when interpreting findings obtained with chemical inhibitors of endocytosis. Moreover, some of these inhibitors are cytotoxic at high concentration.
Relative to classical chemical inhibitors, an advantage of genetic approaches is that they can be more specific than chemical inhibitors in targeting a specific endocytosis mechanism. On the other hand, a drawback of the genetic approach is that it is more time-consuming to deplete an endocytosis-related protein relative to utilizing a chemical approach. In one study using RNAi, the protein downregulation half-life was found to be 40 hr [362]. Another study trying to knock down CHC with RNAi also reported a 48-hr lag time before achieving 80% protein depletion [363]. During the time in which effectors are gradually depleted or an organism is developed as a knockout, compensatory mechanisms can be triggered, leading to confounding effects.
In terms of understanding the role of different pathways in the uptake of DDS, intriguing tools to explore CME have been developed. Disruption of CHC is an efficient and reliable way to switch off CME in vitro, while new mouse models have increasingly enabled studies to explore its role in vivo. In contrast, knockdown of CLC, AP2 and epsin isoforms affect CME in a cargo-dependent manner, and thus may be more applicable to investigations targeted to the specific receptors known to be affected by these manipulations. An attractive new approach is the introduction of chemical or temperature-sensitive switchable systems generated through protein engineering approaches. Many of these approaches can even be applied in vivo in genetically engineered murine models, thus relating in vitro studies to in vivo biodistribution and pharmacokinetics of the DDS.
Genetic mechanisms to impair caveolar endocytosis, macropinocytosis, phagocytosis and noncanonical endocytosis pathways lag far behind approaches to inhibit CME. Some of this lag will be resolved over time, as the study of these endocytotic mechanisms catches up to the work in the CME area, ongoing for 40+ years. Another component of this lag relates to the functional redundancy inherent in these pathways, with multiple caveolin, dynamin and myosin isoforms facilitating these endocytotic functions relative to conserved proteins like CHC. This redundancy requires more complicated generation of research strains or cell lines. In addition, some discrepancies have been observed between the effects of in vitro knockdown of an endocytosis-related protein versus the physiological consequences of in vivo knockout of the same protein, suggesting that the multicellular tissue environment also plays a role in response of depletion of an endocytosis-related protein.
Besides pharmacological and genetic tools, other methods have also been used to identify the endocytosis mechanism of DDS. Colocalization with ligands that are already known to be internalized through a certain pathway can be used to deduce the internalization mechanism of an unknown particle [364]. One classical example is colocalization with fluorescent-dextran, which is known to be internalized through macropinocytosis, and is used to identify uptake by macropinocytosis in vitro and in vivo [365–368]. Colocalization with Tf has been used in some studies to identify CME [369, 370]. Colocalization with cholera toxin B was thought to be a marker for caveolae-mediated endocytosis[70, 371], although studies have shown now that cholera toxin B can be internalized through multiple different pathways [372]. Colocalization with the molecular components of each pathway has also been applied in some studies to identify each pathway. Examples are colocalization with CHC or caveolin-1 to identify uptake by CME and caveolae-mediated endocytosis, respectively [369, 373, 374]. In addition, subcellular fractionation, where subcellular organelles are biochemically isolated and analyzed, has been established [375, 376] and used not only to study endocytic processes, but also the intracellular trafficking of drug carriers or drugs. Subcellular fractionation has been used, for instance, in tracing the intracellular fate of silver nanoparticles and their interaction with cellular proteins [377, 378], assessing drug distribution across different subcellular compartments when delivered by nanoparticles [379], tracking subcellular targeting of fluorescent labeled silicon nanoparticles [380], and in cellular uptake levels and intracellular distribution of Gemini surfactants utilized as non-viral gene delivery vectors [381]. Competitive inhibition assays are also sometimes used to confirm that ligand-conjugated DDS are internalized and trafficked by the same endocytosis pathway as the free ligand [329].
Studies of mechanisms of the endocytosis of particular DDS should be capable of improving their design through identification of critical limiting steps in the release of drug to cytosol. As discussed, relative to chemical inhibitors, genetic methods for probing endocytosis are more specific when they are available for the pathway of interest. Despite this, there is a general lack of application of genetic approaches to mechanistic studies of DDS endocytosis. The emerging intracellular switch approaches which use drug-triggered or thermally responsive mechanisms to introduce rapid, reversible and highly specific changes in CME have significant potential for study of DDS uptake mechanisms. Extension of these approaches in stable cell lines and/or animal models, and the generation of additional tools to target other endocytotic pathways will increase the opportunities for their application to gain additional mechanistic insights into endocytosis and trafficking of DDS.
Acknowledgement
This work was supported by the National Institutes of Health [grant numbers EY011396 and EY026635 to SHA].
Abbreviations:
- AP-2
heterotetrameric adaptor protein-2
- Au-NP
gold nanoparticle
- CAM
cellular adhesion molecules
- CHC
clathrin heavy chain
- CLC
clathrin light chain
- CME
clathrin mediated endocytosis
- DDS
drug delivery systems
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EHD
Eps15 homology domain
- ELP
elastin-like polypeptide
- ER
endoplasmic reticulum
- FR
folate receptor
- GPCR
G-protein coupled receptor
- HUVEC
human umbilical vein endothelial cells
- HER
human epidermal growth factor receptor
- ICAM
intercellular adhesion molecule
- IL-2
interleukin 2
- IL-2R
interleukin 2 receptor
- IVIS
in vivo imaging system
- IVM
Intravital microscopy
- JIP
Jun kinase interacting protein
- LDL
low density lipoprotein
- LDLR
low density lipoprotein receptor
- LSD
Lysosomal storage disease
- PEG
polyethylene glycol
- PEI
poly(ethylenimine)
- PECAM
platelet endothelial cell adhesion molecule
- PV-1
plasmalemma vesicle-associated protein
- RBC
red blood cells
- RES
reticuloendothelial system
- Tf
Transferrin
- TfR
Transferrin Receptor
- VCAM
vascular cell adhesion molecule
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC, Mahmoudi M, Cellular uptake of nanoparticles: journey inside the cell, Chem Soc Rev, 46 (2017) 4218–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Donahue ND, Acar H, Wilhelm S, Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine, Adv Drug Deliv Rev, 143 (2019) 68–96. [DOI] [PubMed] [Google Scholar]
- [3].Foroozandeh P, Aziz AA, Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles, Nanoscale Res Lett, 13 (2018) 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Duncan R, Richardson SC, Endocytosis and intracellular trafficking as gateways for nanomedicine delivery: opportunities and challenges, Mol Pharm, 9 (2012) 2380–2402. [DOI] [PubMed] [Google Scholar]
- [5].Duncan R, Vicent MJ, Polymer therapeutics-prospects for 21st century: The end of the beginning, Adv Drug Deliv Rev, 65 (2013) 60–70. [DOI] [PubMed] [Google Scholar]
- [6].Duncan R, Gaspar R, Nanomedicine(s) under the Microscope, Mol Pharm, 8 (2011) 2101–2141. [DOI] [PubMed] [Google Scholar]
- [7].Mettlen M, Chen PH, Srinivasan S, Danuser G, Schmid SL, Regulation of Clathrin-Mediated Endocytosis, Annu Rev Biochem, 87 (2018) 871–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schmid SL, Sorkin A, Zerial M, Endocytosis: Past, present, and future, Cold Spring Harb Perspect Biol, 6 (2014) a022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dutta D, Donaldson JG, Search for inhibitors of endocytosis: Intended specificity and unintended consequences, Cell Logist, 2 (2012) 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ivanov AI, Pharmacological Inhibition of Endocytic Pathways: Is It Specific Enough to Be Useful?, in: Ivanov AI (Ed.) Exocytosis and Endocytosis, Humana Press, Totowa, NJ, 2008, pp. 15–33. [DOI] [PubMed] [Google Scholar]
- [11].Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K, Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles, Mol Biol Cell, 10 (1999) 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang LH, Rothberg KG, Anderson RG, Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation, J Cell Biol, 123 (1993) 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schlegel R, Dickson RB, Willingham MC, Pastan IH, Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha 2-macroglobulin, Proc Natl Acad Sci U S A, 79 (1982) 2291–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Krausslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V, Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition, Cell, 146 (2011) 471–484. [DOI] [PubMed] [Google Scholar]
- [15].Banbury DN, Oakley JD, Sessions RB, Banting G, Tyrphostin A23 inhibits internalisation of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex, J Bio Chem, (2003). [DOI] [PubMed] [Google Scholar]
- [16].Dejonghe W, Kuenen S, Mylle E, Vasileva M, Keech O, Viotti C, Swerts J, Fendrych M, Ortiz-Morea FA, Mishev K, Delang S, Scholl S, Zarza X, Heilmann M, Kourelis J, Kasprowicz J, Nguyen le SL, Drozdzecki A, Van Houtte I, Szatmari AM, Majda M, Baisa G, Bednarek SY, Robert S, Audenaert D, Testerink C, Munnik T, Van Damme D, Heilmann I, Schumacher K, Winne J, Friml J, Verstreken P, Russinova E, Mitochondrial uncouplers inhibit clathrin-mediated endocytosis largely through cytoplasmic acidification, Nat Comm, 7 (2016) 11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dejonghe W, Sharma I, Denoo B, De Munck S, Lu Q, Mishev K, Bulut H, Mylle E, De Rycke R, Vasileva M, Savatin DV, Nerinckx W, Staes A, Drozdzecki A, Audenaert D, Yperman K, Madder A, Friml J, Van Damme D, Gevaert K, Haucke V, Savvides SN, Winne J, Russinova E, Disruption of endocytosis through chemical inhibition of clathrin heavy chain function, Nat Chem Bio, 15 (2019) 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Elkin SR, Oswald NW, Reed DK, Mettlen M, MacMillan JB, Schmid SL, Ikarugamycin: A Natural Product Inhibitor of Clathrin-Mediated Endocytosis, Traffic (Copenhagen, Denmark), 17 (2016) 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Motley A, Bright NA, Seaman MNJ, Robinson MS Clathrin-mediated endocytosis in AP-2–depleted cells, J Cell Biol, 162 (2003) 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang F, Khvorova A, Marshall W, Sorkin A, Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference, J Bio Chem, 279 (2004) 16657–16661. [DOI] [PubMed] [Google Scholar]
- [21].Ferreira F, Foley M, Cooke A, Cunningham M, Smith G, Woolley R, Henderson G, Kelly E, Mundell S, Smythe E, Endocytosis of G Protein-Coupled Receptors Is Regulated by Clathrin Light Chain Phosphorylation, Curr Bio, 22 (2012) 1361–1370. [DOI] [PubMed] [Google Scholar]
- [22].Bonazzi M, Vasudevan L, Mallet A, Sachse M, Sartori A, Prevost MC, Roberts A, Taner SB, Wilbur JD, Brodsky FM, Cossart P, Clathrin phosphorylation is required for actin recruitment at sites of bacterial adhesion and internalization, J Cell Biol, 195 (2011) 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Poupon V, Girard M, Legendre-Guillemin V, Thomas S, Bourbonniere L, Philie J, Bright NA, McPherson PS, Clathrin light chains function in mannose phosphate receptor trafficking via regulation of actin assembly, Proc Natl Acad Sci U S A, 105 (2008) 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Majeed SR, Vasudevan L, Chen C-Y, Luo Y, Torres JA, Evans TM, Sharkey A, Foraker AB, Wong NML, Esk C, Freeman TA, Moffett A, Keen JH, Brodsky FM, Clathrin light chains are required for the gyrating-clathrin recycling pathway and thereby promote cell migration, Nat Comm, 5 (2014) 3891–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Biancospino M, Buel GR, Niño CA, Maspero E, di Perrotolo RS, Raimondi A, Redlingshöfer L, Weber J, Brodsky FM, Walters KJ, Polo S, Clathrin light chain A drives selective myosin VI recruitment to clathrin-coated pits under membrane tension, Nat Comm, 10 (2019) 4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grant B, Hirsh D, Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte, Mol Biol Cell, 10 (1999) 4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Allen CL, Goulding D, Field MC, Clathrin-mediated endocytosis is essential in Trypanosoma brucei, EMBO J, 22 (2003) 4991–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laboratory TJ, Alleles produced for the KOMP project by The Jackson Laboratory, MGI Direct Data Submission, MGI Direct Data Submission, 2012. [Google Scholar]
- [29].Wu S, Majeed SR, Evans TM, Camus MD, Wong NML, Schollmeier Y, Park M, Muppidi JR, Reboldi A, Parham P, Cyster JG, Brodsky FM, Clathrin light chains’ role in selective endocytosis influences antibody isotype switching, Proc Natl Acad Sci U S A, 113 (2016) 9816–9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mitsunari T, Nakatsu F, Shioda N, Love PE, Grinberg A, Bonifacino JS, Ohno H, Clathrin adaptor AP-2 is essential for early embryonal development, Mol Cell Biol, 25 (2005) 9318–9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen C, Zhuang X, Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus, Proc Natl Acad Sci U S A, 105 (2008) 11790–11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pasula S, Cai X, Dong Y, Messa M, McManus J, Chang B, Liu X, Zhu H, Mansat RS, Yoon SJ, Hahn S, Keeling J, Saunders D, Ko G, Knight J, Newton G, Luscinskas F, Sun X, Towner R, Lupu F, Xia L, Cremona O, De Camilli P, Min W, Chen H, Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling, J Clin Invest, 122 (2012) 4424–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Boucrot E, Pick A, Çamdere G, Liska N, Evergren E, McMahon HT, Kozlov MM, Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains, Cell, 149 (2012) 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Messa M, Fernández-Busnadiego R, Sun EW, Chen H, Czapla H, Wrasman K, Wu Y, Ko G, Ross T, Wendland B, De Camilli P, Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits, Elife, 3 (2014) e03311-e03311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Altschuler Y, Barbas SM, Terlecky LJ, Tang K, Hardy S, Mostov KE, Schmid SL, Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms, J Cell Biol, 143 (1998) 1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sever S, Muhlberg AB, Schmid SL, Impairment of dynamin’s GAP domain stimulates receptor-mediated endocytosis, Nature, 398 (1999) 481–486. [DOI] [PubMed] [Google Scholar]
- [37].Sever S, Damke H, Schmid SL, Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis, J Cell Biol, 150 (2000) 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ferguson SM, Brasnjo G, Hayashi M, Wölfel M, Collesi C, Giovedi S, Raimondi A, Gong L-W, Ariel P, Paradise S, Toole E, Flavell R, Cremona O, Miesenböck G, Ryan TA, De Camilli P, A Selective Activity-Dependent Requirement for Dynamin 1 in Synaptic Vesicle Endocytosis, Science, 316 (2007) 570. [DOI] [PubMed] [Google Scholar]
- [39].Liu Y-W, Surka MC, Schroeter T, Lukiyanchuk V, Schmid SL, Isoform and Splice-Variant Specific Functions of Dynamin-2 Revealed by Analysis of Conditional Knock-Out Cells, Mol Biol Cell, 19 (2008) 5347–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona O, O’ Toole E, De Camilli P, Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits, Dev Cell, 17 (2009) 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Park RJ, Shen H, Liu L, Liu X, Ferguson SM, De Camilli P, Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors, J Cell Sci, 126 (2013) 5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wood LA, Larocque G, Clarke NI, Sarkar S, Royle SJ, New tools for “hot-wiring” clathrin-mediated endocytosis with temporal and spatial precision, J Cell Biol, 216 (2017) 4351–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pastuszka MK, Okamoto CT, Hamm-Alvarez SF, MacKay JA, Flipping the switch on clathrin-mediated endocytosis using thermally responsive protein microdomains, Adv. Funct. Mater, 24 (2014) 5340–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nogueira-Librelotto DR, Codevilla CF, Farooqi A, Rolim CM, Transferrin-Conjugated Nanocarriers as Active-Targeted Drug Delivery Platforms for Cancer Therapy, Current pharmaceutical design, 23 (2017) 454–466. [DOI] [PubMed] [Google Scholar]
- [45].Daniels TR, Bernabeu E, Rodríguez JA, Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera G, Penichet ML, The transferrin receptor and the targeted delivery of therapeutic agents against cancer, Biochim Biophys Acta, 1820 (2012) 291–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Paterson J, Webster CI, Exploiting transferrin receptor for delivering drugs across the blood-brain barrier, Drug Discovery Today: Technologies, 20 (2016) 49–52. [DOI] [PubMed] [Google Scholar]
- [47].Wang J, Tian S, Petros RA, Napier ME, DeSimone JM, The Complex Role of Multivalency in Nanoparticles Targeting the Transferrin Receptor for Cancer Therapies,J Am Chem Soc, 132 (2010) 11306–11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Banerjee D, Liu AP, Voss NR, Schmid SL, Finn MG, Multivalent Display and Receptor-Mediated Endocytosis of Transferrin on Virus-Like Particles, ChemBioChem, 11 (2010) 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Weaver M, Laske DW, Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas,J Neuro-oncol, 65 (2003) 3–13. [DOI] [PubMed] [Google Scholar]
- [50].Head JF, Wang F, Elliott RL, Antineoplastic drugs that interfere with iron metabolism in cancer cells, Adv Enzym Regul, 37 (1997) 147–169. [DOI] [PubMed] [Google Scholar]
- [51].Senzer NN, Matsuno K, Yamagata N, Fujisawa T, Wasserman E, Sutherland W, Sharma S, Phan A, Abstract C36: MBP-426, a novel liposome-encapsulated oxaliplatin, in combination with 5-FU/leucovorin (LV): Phase I results of a Phase I/II study in gastro-esophageal adenocarcinoma, with pharmacokinetics,Mol Cancer Ther, 8 (2009) C36–C36. [Google Scholar]
- [52].Pirollo KF, Nemunaitis J, Leung PK, Nunan R, Adams J, Chang EH, Safety and Efficacy in Advanced Solid Tumors of a Targeted Nanocomplex Carrying the p53 Gene Used in Combination with Docetaxel: A Phase 1b Study, Mol Ther, 24 (2016) 1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Siefker-Radtke A, Zhang XQ, Guo CC, Shen Y, Pirollo KF, Sabir S, Leung C, Leong-Wu C, Ling CM, Chang EH, Millikan RE, Benedict WF, A Phase l Study of a Tumor-targeted Systemic Nanodelivery System, SGT-94, in Genitourinary Cancers, Mol Ther, 24 (2016) 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A, Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles, Nature, 464 (2010) 1067–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tseng CL, Su WY, Yen KC, Yang KC, Lin FH, The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation, Biomaterials, 30 (2009) 3476–3485. [DOI] [PubMed] [Google Scholar]
- [56].Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H, EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma, Cancer Res, 70 (2010) 6303–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Karyagina TS, Ulasov AV, Slastnikova TA, Rosenkranz AA, Lupanova TN, Khramtsov YV, Georgiev GP, Sobolev AS, Targeted Delivery of 111In Into the Nuclei of EGFR Overexpressing Cells via Modular Nanotransporters With Anti-EGFR Affibody, Front Pharmacol, 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Roncato F, Rruga F, Porcù E, Casarin E, Ronca R, Maccarinelli F, Realdon N, Basso G, Alon R, Viola G, Morpurgo M, Improvement and extension of anti-EGFR targeting in breast cancer therapy by integration with the Avidin-Nucleic-Acid-Nano-Assemblies, Nat Comm, 9 (2018) 4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF, Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances, Adv Drug Deliv Rev, 65 (2013) 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Parton RG, Caveolae: Structure, Function, and Relationship to Disease, Annu Rev Cell Dev Biol, 34 (2018) 111–136. [DOI] [PubMed] [Google Scholar]
- [61].Yeow I, Howard G, Chadwick J, Mendoza-Topaz C, Hansen CG, Nichols BJ, Shvets E, EHD Proteins Cooperate to Generate Caveolar Clusters and to Maintain Caveolae during Repeated Mechanical Stress, Curr Biol, 27 (2017) 2951–2962 e2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Moren B, Shah C, Howes MT, Schieber NL, McMahon HT, Parton RG, Daumke O, Lundmark R, EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization, Mol Biol Cell, 23 (2012) 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mayor S, Parton RG, Donaldson JG, Clathrin-independent pathways of endocytosis, Cold Spring Harb Perspect Biol, 6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pelkmans L, Kartenbeck J, Helenius A, Caveolar endocytosis of simian virus 40 reveals a new twostep vesicular-transport pathway to the ER, Nat Cell Biol, 3 (2001) 473–483. [DOI] [PubMed] [Google Scholar]
- [65].Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A, Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation, J Cell Biol, 191 (2010) 615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pelkmans L, Burli T, Zerial M, Helenius A, Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic, Cell, 118 (2004) 767–780. [DOI] [PubMed] [Google Scholar]
- [67].Rothberg KG, Ying YS, Kamen BA, Anderson RG, Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate, J Cell Biol, 111 (1990) 2931–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Williams TM, Lisanti MP, The caveolin proteins, Genome Biol, 5 (2004) 214–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nichols BJ, A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex, Nat Cell Bio, 4 (2002) 374–378. [DOI] [PubMed] [Google Scholar]
- [70].Orlandi PA, Fishman PH, Filipin-dependent Inhibition of Cholera Toxin: Evidence for Toxin Internalization and Activation through Caveolae-like Domains, J Cell Biol, 141 (1998) 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV, Loss of Caveolae, Vascular Dysfunction, and Pulmonary Defects in Caveolin-1 Gene-Disrupted Mice, Science, 293 (2001) 2449. [DOI] [PubMed] [Google Scholar]
- [72].Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP, Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo, J Bio Chem, 276 (2001) 48619–48622. [DOI] [PubMed] [Google Scholar]
- [73].Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Knietz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP, Caveolin-1 null mice are viable, but show evidence of hyper-proliferative and vascular abnormalities, J Bio Chem, (2001). [DOI] [PubMed] [Google Scholar]
- [74].Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H Jr., Christ GJ, Edelmann W, Lisanti MP, Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae, Mol Cell Biol, 22 (2002) 2329–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Kneitz B, Edelmann W, Lisanti MP, Caveolin-3 Null Mice Show a Loss of Caveolae, Changes in the Microdomain Distribution of the Dystrophin-Glycoprotein Complex, and T-tubule Abnormalities, J Bio Chem, 276 (2001) 21425–21433. [DOI] [PubMed] [Google Scholar]
- [76].Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF, Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance, Cell Metab, 8 (2008) 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hansen CG, Shvets E, Howard G, Riento K, Nichols BJ, Deletion of cavin genes reveals tissue-specific mechanisms for morphogenesis of endothelial caveolae, Nat Comm, 4 (2013) 1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ogata T, Naito D, Nakanishi N, Hayashi YK, Taniguchi T, Miyagawa K, Hamaoka T, Maruyama N, Matoba S, Ikeda K, Yamada H, Oh H, Ueyama T, MURC/Cavin-4 facilitates recruitment of ERK to caveolae and concentric cardiac hypertrophy induced by α1-adrenergic receptors, Proc Natl Acad Sci U S A, 111 (2014) 3811–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rippe B, Rosengren BI, Carlsson O, Venturoli D, Transendothelial transport: the vesicle controversy, J Vasc Res, 39 (2002) 375–390. [DOI] [PubMed] [Google Scholar]
- [80].Niles WD, Malik AB, Endocytosis and exocytosis events regulate vesicle traffic in endothelial cells, J Membr Biol, 167 (1999) 85–101. [DOI] [PubMed] [Google Scholar]
- [81].Predescu D, Palade GE, Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium, Am J Physiol, 265 (1993) H725–733. [DOI] [PubMed] [Google Scholar]
- [82].Schnitzer JE, Oh P, Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins, J Bio Chem, 269 (1994) 6072–6082. [PubMed] [Google Scholar]
- [83].Schnitzer JE, Allard J, Oh P, NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia, Am J Physiol, 268 (1995) H48–55. [DOI] [PubMed] [Google Scholar]
- [84].Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB, Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway, J Cell Biol, 150 (2000) 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Parton RG, Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae, J Histochem Cytochem, 42 (1994) 155–166. [DOI] [PubMed] [Google Scholar]
- [86].Schnitzer JE, Oh P, Pinney E, Allard J, Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules, J Cell Biol, 127 (1994) 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Thomsen P, Roepstorff K, Stahlhut M, van Deurs B, Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking, Mol Biol Cell, 13 (2002) 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE, Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung, Nat Biotechnol, 25 (2007) 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rosengren BI, Rippe A, Rippe C, Venturoli D, Sward K, Rippe B, Transvascular protein transport in mice lacking endothelial caveolae, Am J Physiol Heart Circ Physiol, 291 (2006) H1371–1377. [DOI] [PubMed] [Google Scholar]
- [90].Rippe B, Taylor A, NEM and filipin increase albumin transport in lung microvessels, Am J Physiol Heart Circ Physiol, 280 (2001) H34–41. [DOI] [PubMed] [Google Scholar]
- [91].Rosengren BI, Al Rayyes O, Rippe B, Transendothelial transport of low-density lipoprotein and albumin across the rat peritoneum in vivo: effects of the transcytosis inhibitors NEM and filipin, J Vasc Res, 39 (2002) 230–237. [DOI] [PubMed] [Google Scholar]
- [92].Ogawa K, Imai M, Ogawa T, Tsukamoto Y, Sasaki F, Caveolar and intercellular channels provide major transport pathways of macromolecules across vascular endothelial cells, Anat Rec, 264 (2001) 32–42. [DOI] [PubMed] [Google Scholar]
- [93].Damm EM, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A, Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae, J Cell Biol, 168 (2005) 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang Z, Tiruppathi C, Cho J, Minshall RD, Malik AB, Delivery of nanoparticle: complexed drugs across the vascular endothelial barrier via caveolae, IUBMB Life, 63 (2011) 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE, Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy, Nature, 429 (2004) 629–635. [DOI] [PubMed] [Google Scholar]
- [96].Wang Z, Tiruppathi C, Minshall RD, Malik AB, Size and dynamics of caveolae studied using nanoparticles in living endothelial cells, ACS Nano, 3 (2009) 4110–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yin Q, Tang L, Cai K, Yang X, Yin L, Zhang Y, Dobrucki LW, Helferich WG, Fan TM, Cheng J, Albumin as a “Trojan Horse” for polymeric nanoconjugate transendothelial transport across tumor vasculatures for improved cancer targeting, Biomater Sci, 6 (2018) 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Li F, Zhao Y, Mao C, Kong Y, Ming X, RGD-Modified Albumin Nanoconjugates for Targeted Delivery of a Porphyrin Photosensitizer, Mol Pharm, 14 (2017) 2793–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang L, Yang X, Lv Y, Xin X, Qin C, Han X, Yang L, He W, Yin L, Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer, Sci Rep, 7 (2017) 46186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R, Labao E, Hawkins M, Soon-Shiong P, Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel, Clin Cancer Res, 12 (2006) 1317–1324. [DOI] [PubMed] [Google Scholar]
- [101].Liu Z, Chen X, Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy, Chem Soc Rev, 45 (2016) 1432–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Chrastina A, Valadon P, Massey KA, Schnitzer JE, Lung vascular targeting using antibody to aminopeptidase P: CT-SPECT imaging, biodistribution and pharmacokinetic analysis, J Vasc Res, 47 (2010) 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bhushan B, Khanadeev V, Khlebtsov B, Khlebtsov N, Gopinath P, Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery, Adv Colloid Interface Sci, 246 (2017) 13–39. [DOI] [PubMed] [Google Scholar]
- [104].Larsen MT, Kuhlmann M, Hvam ML, Howard KA, Albumin-based drug delivery: harnessing nature to cure disease, Mol Cell Ther, 4 (2016) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Guo L, Zhang H, Hou Y, Wei T, Liu J, Plasmalemma vesicle-associated protein: A crucial component of vascular homeostasis, Exp Ther Med, 12 (2016) 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Marchetti GM, Burwell TJ, Peterson NC, Cann JA, Hanna RN, Li Q, Ongstad EL, Boyd JT, Kennedy MA, Zhao W, Rickert KW, Grimsby JS, Dall’Acqua WF, Wu H, Tsui P, Borrok MJ, Gupta R, Targeted drug delivery via caveolae-associated protein PV1 improves lung fibrosis, Commun Biol, 2 (2019) 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Stan RV, Structure of caveolae, Biochim Biophys Acta, 1746 (2005) 334–348. [DOI] [PubMed] [Google Scholar]
- [108].Li Q, Peterson N, Hanna RN, Kuszpit K, White J, Allen KL, Barnes A, Rickert KW, Shan L, Wu H, Dall’Acqua WF, Tsui P, Borrok MJ, Antibody Fragment F(ab’)2 Targeting Caveolae-Associated Protein PV1 for Selective Kidney Targeting and Retention, Mol Pharm, 17 (2020) 507–516. [DOI] [PubMed] [Google Scholar]
- [109].Canton J, Macropinocytosis: New Insights Into Its Underappreciated Role in Innate Immune Cell Surveillance, Front Immunol, 9 (2018) 2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Egami Y, Taguchi T, Maekawa M, Arai H, Araki N, Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation, Front Physiol, 5 (2014) 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Porat-Shliom N, Kloog Y, Donaldson JG, A unique platform for H-Ras signaling involving clathrin-independent endocytosis, Mol Biol Cell, 19 (2008) 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S, Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling, J Cell Biol, 188 (2010) 547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Araki N, Johnson MT, Swanson JA, A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages, J Cell Biol, 135 (1996) 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lin HP, Singla B, Ghoshal P, Faulkner JL, Cherian-Shaw M, O’Connor PM, She JX, Belin de Chantemele EJ, Csanyi G, Identification of novel macropinocytosis inhibitors using a rational screen of Food and Drug Administration-approved drugs, Br J Pharmacol, 175 (2018) 3640–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Stewart CM, Dorion SS, Ottenbrite MAF, LeBlond ND, Smith TKT, Qiu S, Fullerton MD, Kobasa D, Cote M, A Diacylglycerol Kinase Inhibitor, R-59–022, Blocks Filovirus Internalization in Host Cells, Viruses, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Thu PM, Zheng Z-G, Zhou Y-P, Wang Y-Y, Zhang X, Jing D, Cheng H-M, Li J, Li P, Xu X, Phellodendrine chloride suppresses proliferation of KRAS mutated pancreatic cancer cells through inhibition of nutrients uptake via macropinocytosis, Eur J Pharmacol, 850 (2019) 23–34. [DOI] [PubMed] [Google Scholar]
- [117].Foth BJ, Goedecke MC, Soldati D, New insights into myosin evolution and classification, Proc Natl Acad Sci U S A, 103 (2006) 3681–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J, Myosin: The Actin Motor Protein, Molecular Cell Biology. 4th edition, WH Freeman; 2000. [Google Scholar]
- [119].Novak KD, Peterson MD, Reedy MC, Titus MA, Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis, J Cell Biol, 131 (1995) 1205–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Brandstaetter H, Kendrick-Jones J, Buss F, Myo1c regulates lipid raft recycling to control cell spreading, migration and <em>Salmonella</em> invasion, J Cell Sci, 125 (2012) 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jiang J, Kolpak AL, Bao Z-Z, Myosin IIB isoform plays an essential role in the formation of two distinct types of macropinosomes, Cytoskeleton (Hoboken), 67 (2010) 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Chabaud M, Heuzé ML, Bretou M, Vargas P, Maiuri P, Solanes P, Maurin M, Terriac E, Le Berre M, Lankar D, Piolot T, Adelstein RS, Zhang Y, Sixt M, Jacobelli J, Bénichou O, Voituriez R, Piel M, Lennon-Duménil A-M, Cell migration and antigen capture are antagonistic processes coupled by myosin II in dendritic cells, Nat Comm, 6 (2015) 7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Amyere M, Payrastre B, Krause U, Smissen PVD, Veithen A, Courtoy PJ, Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C, Mol Biol Cell, 11 (2000) 3453–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Veithen A, Cupers P, Baudhuin P, Courtoy PJ, v-Src induces constitutive macropinocytosis in rat fibroblasts, J Cell Sci, 109 (1996) 2005–2012. [DOI] [PubMed] [Google Scholar]
- [125].West MA, Bretscher MS, Watts C, Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells, J Cell Biol, 109 (1989) 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Williamson CD, Donaldson JG, Arf6, JIP3, and dynein shape and mediate macropinocytosis, Mol Biol Cell, 30 (2019) 1477–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DWY, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG, Lipid-like materials for low-dose, in vivo gene silencing, Proc Natl Acad Sci U S A, 107 (2010) 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, Karagiannis E, Love K, Chen D, Zoncu R, Buganim Y, Schroeder A, Langer R, Anderson DG, Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling, Nat Biotechnol, 31 (2013) 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Dong Y, Love KT, Dorkin JR, Sirirungruang S, Zhang Y, Chen D, Bogorad RL, Yin H, Chen Y, Vegas AJ, Alabi CA, Sahay G, Olejnik KT, Wang W, Schroeder A, Lytton-Jean AKR, Siegwart DJ, Akinc A, Barnes C, Barros SA, Carioto M, Fitzgerald K, Hettinger J, Kumar V, Novobrantseva TI, Qin J, Querbes W, Koteliansky V, Langer R, Anderson DG, Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates, Proc Natl Acad Sci U S A, 111 (2014) 3955–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Goncalves C, Mennesson E, Fuchs R, Gorvel JP, Midoux P, Pichon C, Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells, Mol Ther, 10 (2004) 373–385. [DOI] [PubMed] [Google Scholar]
- [131].Walsh M, Tangney M, O’Neill MJ, Larkin JO, Soden DM, McKenna SL, Darcy R, O’Sullivan GC, O’Driscoll CM, Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-L-lysine compacted DNA: implications for cancer gene therapy, Mol Pharm, 3 (2006) 644–653. [DOI] [PubMed] [Google Scholar]
- [132].Peddi S, Pan X, MacKay JA, Intracellular Delivery of Rapamycin From FKBP Elastin-Like Polypeptides Is Consistent With Macropinocytosis, Front Pharmacol, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SEL, Vader P, Extracellular vesicles as drug delivery systems: Why and how?, Adv Drug Deliv Rev, (2020). [DOI] [PubMed] [Google Scholar]
- [134].Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T, Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes,Sci Rep, 5 (2015) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, Kabanov AV, Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain, Biomaterials, 142 (2017) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Tian T, Zhu Y-L, Zhou Y-Y, Liang G-F, Wang Y-Y, Hu F-H, Xiao Z-D, Exosome Uptake through Clathrin-mediated Endocytosis and Macropinocytosis and Mediating miR-21 Delivery, J Bio Chem, 289 (2014) 22258–22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Khalil IA, Kogure K, Futaki S, Harashima H, High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression, J Bio Chem, 281 (2006) 3544–3551. [DOI] [PubMed] [Google Scholar]
- [138].Nakase I, Noguchi K, Aoki A, Takatani-Nakase T, Fujii I, Futaki S, Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery, Sci Rep, 7 (2017) 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Freeman SA, Grinstein S, Phagocytosis: receptors, signal integration, and the cytoskeleton, Immunol Rev, 262 (2014) 193–215. [DOI] [PubMed] [Google Scholar]
- [140].Axline SG, Reaven EP, Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments, J Cell Biol, 62 (1974) 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sung SJ, Silverstein SC, Inhibition of macrophage phagocytosis by methylation inhibitors. Lack of correlation of protein carboxymethylation and phospholipid methylation with phagocytosis, J Bio Chem, 260 (1985) 546–554. [PubMed] [Google Scholar]
- [142].Magae J, Nagi T, Takaku K, Kataoka T, Koshino H, Uramoto M, Nagai K, Screening for specific inhibitors of phagocytosis of thioglycollate-elicited macrophages, Biosci Biotech Bioch, 58 (1994) 104–107. [DOI] [PubMed] [Google Scholar]
- [143].de Oliveira CA, Mantovani B, Latrunculin A is a potent inhibitor of phagocytosis by macrophages, Life sciences, 43 (1988) 1825–1830. [DOI] [PubMed] [Google Scholar]
- [144].Rampersad GC, Suck G, Sakac D, Fahim S, Foo A, Denomme GA, Langler RF, Branch DR, Chemical compounds that target thiol-disulfide groups on mononuclear phagocytes inhibit immune mediated phagocytosis of red blood cells, Transfusion, 45 (2005) 384–393. [DOI] [PubMed] [Google Scholar]
- [145].Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, van Rooijen N, Weissman IL, CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells, Cell, 138 (2009) 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL, PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity, Nature, 545 (2017) 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM, McKenna KM, Ho PY, Cheng RZ, Chen JY, Barkal LJ, Ring AM, Weissman IL, Maute RL, Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy, Nat Immunol, 19 (2018) 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal LJ, Weissman IL, CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy, Nature, 572 (2019) 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hong Y, Ghebrehiwet B, Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3, Clin Immunol Immunopathol, 62 (1992) 133–138. [DOI] [PubMed] [Google Scholar]
- [150].Rooijakkers SHM, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJB, van Kessel KPM, van Strijp JAG, Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases, Nat Immunol, 6 (2005) 920–927. [DOI] [PubMed] [Google Scholar]
- [151].Prasadarao NV, Blom AM, Villoutreix BO, Linsangan LC, A Novel Interaction of Outer Membrane Protein A with C4b Binding Protein Mediates Serum Resistance of <em>Escherichia coli</em> K1, J Immunol, 169 (2002) 6352. [DOI] [PubMed] [Google Scholar]
- [152].Davis JM, Rasmussen SB, O’Brien AD, Cytotoxic Necrotizing Factor Type 1 Production by Uropathogenic Escherichia coli Modulates Polymorphonuclear Leukocyte Function,Infect Immun, 73 (2005) 5301–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Garrity-Ryan L, Kazmierczak B, Kowal R, Comolli J, Hauser A, Engel JN, The Arginine Finger Domain of ExoT Contributes to Actin Cytoskeleton Disruption and Inhibition of Internalization ofPseudomonas aeruginosa by Epithelial Cells and Macrophages,Infect Immun, 68 (2000) 7100–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wheeler RT, Kombe D, Agarwala SD, Fink GR, Dynamic, Morphotype-Specific Candida albicans β-Glucan Exposure during Infection and Drug Treatment, PLOS Pathogens, 4 (2008) e1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bose I, Reese AJ, Ory JJ, Janbon G, Doering TL, A Yeast under Cover: the Capsule of Cryptococcus neoformans, Eukaryotic Cell, 2 (2003) 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Haney MS, Bohlen CJ, Morgens DW, Ousey JA, Barkal AA, Tsui CK, Ego BK, Levin R, Kamber RA, Collins H, Tucker A, Li A, Vorselen D, Labitigan L, Crane E, Boyle E, Jiang L, Chan J, Rincón E, Greenleaf WJ, Li B, Snyder MP, Weissman IL, Theriot JA, Collins SR, Barres BA, Bassik MC, Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens, Nat Genet, 50 (2018) 1716–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ebensen T, Paukner S, Link C, Kudela P, de Domenico C, Lubitz W, Guzmán CA, Bacterial Ghosts Are an Efficient Delivery System for DNA Vaccines, J Immunol, 172 (2004) 6858. [DOI] [PubMed] [Google Scholar]
- [158].Paukner S, Kudela P, Kohl G, Schlapp T, Friedrichs S, Lubitz W, DNA-Loaded Bacterial Ghosts Efficiently Mediate Reporter Gene Transfer and Expression in Macrophages, Mol Ther, 11 (2005) 215–223. [DOI] [PubMed] [Google Scholar]
- [159].Mayr UB, Walcher P, Azimpour C, Riedmann E, Haller C, Lubitz W, Bacterial ghosts as antigen delivery vehicles, Adv Drug Deliv Rev, 57 (2005) 1381–1391. [DOI] [PubMed] [Google Scholar]
- [160].Jiao H, Yang H, Zhao D, Chen J, Zhang Q, Liang J, Yin Y, Kong G, Li G, Design and immune characterization of a novel Neisseria gonorrhoeae DNA vaccine using bacterial ghosts as vector and adjuvant, Vaccine, 36 (2018) 4532–4539. [DOI] [PubMed] [Google Scholar]
- [161].Hou R, Li M, Tang T, Wang R, Li Y, Xu Y, Tang L, Wang L, Liu M, Jiang Y, Cui W, Qiao X, Construction of Lactobacillus casei ghosts by Holin-mediated inactivation and the potential as a safe and effective vehicle for the delivery of DNA vaccines, BMC Microbiol, 18 (2018) 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Aouadi M, Tencerova M, Vangala P, Yawe JC, Nicoloro SM, Amano SU, Cohen JL, Czech MP, Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice, Proc Natl Acad Sci U S A, 110 (2013) 8278–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, Ostroff GR, Czech MP, Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation, Nature, 458 (2009) 1180–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Serafini S, Rossi L, Antonelli A, Fraternale A, Cerasi A, Crinelli R, Chiarantini L, Schiavano GF, Magnani M, Drug Delivery through Phagocytosis of Red Blood Cells, Transfus Med Hemother, 31 (2004) 92–101. [Google Scholar]
- [165].Rossi L, Brandi G, Schiavano GF, Balestra E, Millo E, Scarfi S, Damonte G, Gasparini A, Magnani M, Perno CF, Benatti U, De Flora A, Macrophage protection against human immunodeficiency virus or herpes simplex virus by red blood cell-mediated delivery of a heterodinucleotide of azidothymidine and acyclovir,AIDS Res Hum Retrov, 14 (1998) 435–444. [DOI] [PubMed] [Google Scholar]
- [166].Crinelli R, Antonelli A, Bianchi M, Gentilini L, Scaramucci S, Magnani M, Selective inhibition of NF-kB activation and TNF-alpha production in macrophages by red blood cell-mediated delivery of dexamethasone, Blood Cell Mol Dis, 26 (2000) 211–222. [DOI] [PubMed] [Google Scholar]
- [167].Rossi L, Serafini S, Cenerini L, Picardi F, Bigi L, Panzani I, Magnani M, Erythrocyte-mediated delivery of dexamethasone in patients with chronic obstructive pulmonary disease, Biotechnol Appl Biochem, 33 (2001) 85–89. [DOI] [PubMed] [Google Scholar]
- [168].Castro M, Rossi L, Papadatou B, Bracci F, Knafelz D, Ambrosini MI, Calce A, Serafini S, Isacchi G, D’Orio F, Mambrini G, Magnani M, Long-term treatment with autologous red blood cells loaded with dexamethasone 21-phosphate in pediatric patients affected by steroid-dependent Crohn disease, J Pediatr Gastr Nutr, 44 (2007) 423–426. [DOI] [PubMed] [Google Scholar]
- [169].Chessa L, Leuzzi V, Plebani A, Soresina A, Micheli R, D’Agnano D, Venturi T, Molinaro A, Fazzi E, Marini M, Ferremi Leali P, Quinti I, Cavaliere FM, Girelli G, Pietrogrande MC, Finocchi A, Tabolli S, Abeni D, Magnani M, Intra-Erythrocyte Infusion of Dexamethasone Reduces Neurological Symptoms in Ataxia Teleangiectasia Patients: Results of a Phase 2 Trial, Orphanet J Rare Dis, 9 (2014) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Hunault-Berger M, Leguay T, Huguet F, Leprêtre S, Deconinck E, Ojeda-Uribe M, Bonmati C, Escoffre-Barbe M, Bories P, Himberlin C, Chevallier P, Rousselot P, Reman O, Boulland M-L, Lissandre S, Turlure P, Bouscary D, Sanhes L, Legrand O, Lafage-Pochitaloff M, Béné MC, Liens D, Godfrin Y, Ifrah N, Dombret H, Leukemia G.f.R.o.A.A.L., A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: The GRASPALL/GRAALL-SA2–2008 study, Am J Hematol, 90 (2015) 811–818. [DOI] [PubMed] [Google Scholar]
- [171].Godfrin Y, Thomas X, Bertrand Y, Duget C, L-Asparaginase Loaded into Erythrocytes (GRASPA): Principle and Interests in Acute Lymphoblastic Leukemia, Blood, 110 (2007) 4325–4325. [Google Scholar]
- [172].Hammel P, Fabienne P, Mineur L, Metges JP, Andre T, De La Fouchardiere C, Louvet C, El Hajbi F, Faroux R, Guimbaud R, Tougeron D, Bouche O, Lecomte T, Rebischung C, Tournigand C, Cros J, Kay R, Hamm A, Gupta A, Bachet JB, El Hariry I, Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial, Eur J Cancer (Oxford, England : 1990), 124 (2020) 91–101. [DOI] [PubMed] [Google Scholar]
- [173].Bourgeaux V, Aufradet E, Campion Y, De Souza G, Horand F, Bessaad A, Chevrier A-M, Canet-Soulas E, Godfrin Y, Martin C, Efficacy of homologous inositol hexaphosphate-loaded red blood cells in sickle transgenic mice, Br J Haematol, 157 (2012) 357–369. [DOI] [PubMed] [Google Scholar]
- [174].Protasov ES, Borsakova DV, Alexandrovich YG, Korotkov AV, Kosenko EA, Butylin AA, Ataullakhanov FI, Sinauridze EI, Erythrocytes as bioreactors to decrease excess ammonium concentration in blood,Sci Rep, 9 (2019) 1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Kawamura K, Kadowaki N, Suzuki R, Udagawa S, Kasaoka S, Utoguchi N, Kitawaki T, Sugimoto N, Okada N, Maruyama K, Uchiyama T, Dendritic Cells That Endocytosed Antigen-Containing IgG-Liposomes Elicit Effective Antitumor Immunity, J Immunother, 29 (2006) 165–174. [DOI] [PubMed] [Google Scholar]
- [176].Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Hiroshi Morisaki J, Kim J, Park S, Darwish M, Lee B-C, Hernandez H, Loyet KM, Lupardus P, Fong R, Yan D, Chalouni C, Luis E, Khalfin Y, Plise E, Cheong J, Lyssikatos JP, Strandh M, Koefoed K, Andersen PS, Flygare JA, Wah Tan M, Brown EJ, Mariathasan S, Novel antibody–antibiotic conjugate eliminates intracellular S. aureus, Nature, 527 (2015) 323–328. [DOI] [PubMed] [Google Scholar]
- [177].Li H, Tatematsu K, Somiya M, Iijima M, Kuroda S.i., Development of a macrophage-targeting and phagocytosis-inducing bio-nanocapsule-based nanocarrier for drug delivery, Acta Biomaterialia, 73 (2018) 412–423. [DOI] [PubMed] [Google Scholar]
- [178].Kimura H, Ogura Y, Moritera T, Honda Y, Tabata Y, Ikada Y, In vitro phagocytosis of polylactide microspheres by retinal pigment epithelial cells and intracellular drug release, Curr Eye Res, 13 (1994) 353–360. [DOI] [PubMed] [Google Scholar]
- [179].Ogura Y, Kimura H, Biodegradable polymer microspheres for targeted drug delivery to the retinal pigment epithelium, Survey of ophthalmology, 39 Suppl 1 (1995) S17–24. [DOI] [PubMed] [Google Scholar]
- [180].Tuovinen L, Ruhanen E, Kinnarinen T, Ronkko S, Pelkonen J, Urtti A, Peltonen S, Jarvinen K, Starch acetate microparticles for drug delivery into retinal pigment epithelium-in vitro study,J Control Release, 98 (2004) 407–413. [DOI] [PubMed] [Google Scholar]
- [181].Mahajan V, Gaymalov Z, Alakhova D, Gupta R, Zucker IH, Kabanov AV, Horizontal gene transfer from macrophages to ischemic muscles upon delivery of naked DNA with Pluronic block copolymers, Biomaterials, 75 (2016) 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Wayne EC, Long C, Haney MJ, Batrakova EV, Leisner TM, Parise LV, Kabanov AV, Targeted Delivery of siRNA Lipoplexes to Cancer Cells Using Macrophage Transient Horizontal Gene Transfer, Adv Sci (Weinheim, Baden-Wurttemberg, Germany), 6 (2019) 1900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Zhao Y, Haney MJ, Jin YS, Uvarov O, Vinod N, Lee YZ, Langworthy B, Fine JP, Rodriguez M, El-Hage N, Kabanov AV, Batrakova EV, GDNF-expressing macrophages restore motor functions at a severe late-stage, and produce long-term neuroprotective effects at an early-stage of Parkinson’s disease in transgenic Parkin Q311X(A) mice, J Control Release, 315 (2019) 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL, Kabanov AV, Batrakova EV, Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy, J Neuroimmun Pharmacol, (2019). [DOI] [PubMed] [Google Scholar]
- [185].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV, Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells,Nanomed-Nanotechnol, 12 (2016) 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Haney MJ, Klyachko NL, Harrison EB, Zhao Y, Kabanov AV, Batrakova EV, TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease, Adv Healthc Mater, 8 (2019) 1801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Otsuka H, Nagasaki Y, Kataoka K, PEGylated nanoparticles for biological and pharmaceutical applications, Adv Drug Deliv Rev, 55 (2003) 403–419. [DOI] [PubMed] [Google Scholar]
- [188].Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg P-A, Michalak M, Henson PM, Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through trans-Activation of LRP on the Phagocyte, Cell, 123 (2005) 321–334. [DOI] [PubMed] [Google Scholar]
- [189].Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg P-A, Iizuka K, Yokoyama WM, Taylor PA, Cd47 (Integrin-Associated Protein) Engagement of Dendritic Cell and Macrophage Counterreceptors Is Required to Prevent the Clearance of Donor Lymphohematopoietic Cells, J Exp Med, 194 (2001) 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R, CD47 functions as a molecular switch for erythrocyte phagocytosis, Blood, 119 (2012) 5512–5521. [DOI] [PubMed] [Google Scholar]
- [191].Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE, Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles, Science, 339 (2013) 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Sosale NG, Ivanovska II, Tsai RK, Swift J, Hsu JW, Alvey CM, Zoltick PW, Discher DE, “Marker of Self” CD47 on lentiviral vectors decreases macrophage-mediated clearance and increases delivery to SIRPA-expressing lung carcinoma tumors, Mol Ther, 3 (2016) 16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Jalil AR, Andrechak JC, Discher DE, Macrophage checkpoint blockade: results from initial clinical trials, binding analyses, and CD47-SIRPα structure–function, Antib Ther, 3 (2020) 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M, A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1, J Cell Sci, 116 (2003) 1599–1609. [DOI] [PubMed] [Google Scholar]
- [195].Glassman PM, Myerson JW, Ferguson LT, Kiseleva RY, Shuvaev VV, Brenner JS, Muzykantov VR, Targeting drug delivery in the vascular system: Focus on endothelium, Adv Drug Deliv Rev, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ghaffarian R, Muro S, Distinct Subcellular Trafficking Resulting from Monomeric vs Multimeric Targeting to Endothelial ICAM-1: Implications for Drug Delivery, Mol Pharm, 11 (2014) 4350–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Garnacho C, Dhami R, Simone E, Dziubla T, Leferovich J, Schuchman EH, Muzykantov V, Muro S, Delivery of Acid Sphingomyelinase in Normal and Niemann-Pick Disease Mice Using Intercellular Adhesion Molecule-1-Targeted Polymer Nanocarriers, J Pharmacol Exp Ther, 325 (2008) 400–408. [DOI] [PubMed] [Google Scholar]
- [198].Hsu J, Serrano D, Bhowmick T, Kumar K, Shen Y, Kuo YC, Garnacho C, Muro S, Enhanced endothelial delivery and biochemical effects of α-galactosidase by ICAM-1-targeted nanocarriers for Fabry disease, J Control Release, 149 (2011) 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Muro S, New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders, WIREs Nanomed-Nanobiotechnol, 2 (2010) 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Rappaport J, Manthe RL, Garnacho C, Muro S, Altered Clathrin-Independent Endocytosis in Type A Niemann-Pick Disease Cells and Rescue by ICAM-1-Targeted Enzyme Delivery, Mol Pharm, 12 (2015) 1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Solomon M, Muro S, Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives, Adv Drug Deliv Rev, 118 (2017) 109–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Muro S, Mateescu M, Gajewski C, Robinson M, Muzykantov VR, Koval M, Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins, Am J Physiol-Lung Cell Mol Physiol, 290 (2006) L809–L817. [DOI] [PubMed] [Google Scholar]
- [203].Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR, PECAM-targeted delivery of SOD inhibits endothelial inflammatory response, The FASEB Journal, 25 (2011) 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Spencer NY, Engelhardt JF, The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies, Biochemistry, 53 (2014) 1551–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Shuvaev VV, Kiseleva RY, Arguiri E, Villa CH, Muro S, Christofidou-Solomidou M, Stan RV, Muzykantov VR, Targeting superoxide dismutase to endothelial caveolae profoundly alleviates inflammation caused by endotoxin, J Control Release, 272 (2018) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Frick SU, Domogalla MP, Baier G, Wurm FR, Mailänder V, Landfester K, Steinbrink K, Interleukin-2 Functionalized Nanocapsules for T Cell-Based Immunotherapy, ACS Nano, 10 (2016) 9216–9226. [DOI] [PubMed] [Google Scholar]
- [207].Konigsberg PJ, Godtel R, Kissel T, Richer LL, The development of IL-2 conjugated liposomes for therapeutic purposes, Biochimica et Biophysica Acta (BBA) - Biomembranes, 1370 (1998) 243–251. [DOI] [PubMed] [Google Scholar]
- [208].Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ, In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes,J Control Release, 172 (2013) 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Boucrot E, Ferreira APA, Almeida-Souza L, Debard S, Vallis Y, Howard G, Bertot L, Sauvonnet N, McMahon HT, Endophilin marks and controls a clathrin-independent endocytic pathway, Nature, 517 (2015) 460–465. [DOI] [PubMed] [Google Scholar]
- [210].Grassart A, Dujeancourt A, Lazarow PB, Dautry-Varsat A, Sauvonnet N, Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2, EMBO Rep, 9 (2008) 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Simone EA, Zern BJ, Chacko A-M, Mikitsh JL, Blankemeyer ER, Muro S, Stan RV, Muzykantov VR, Endothelial targeting of polymeric nanoparticles stably labeled with the PET imaging radioisotope iodine-124, Biomaterials, 33 (2012) 5406–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, Alder S, Christofidou-Solomidou M, Haut E, Murciano JC, Nakada M, Albelda SM, Muzykantov VR, Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature, J Pharmacol Exp Ther, 300 (2002) 777–786. [DOI] [PubMed] [Google Scholar]
- [213].Rossin R, Muro S, Welch MJ, Muzykantov VR, Schuster DP, In vivo imaging of 64Cu-labeled polymer nanoparticles targeted to the lung endothelium,J Nucl Med, 49 (2008) 103–111. [DOI] [PubMed] [Google Scholar]
- [214].Marcos-Contreras OA, Greineder CF, Kiseleva RY, Parhiz H, Walsh LR, Zuluaga-Ramirez V, Myerson JW, Hood ED, Villa CH, Tombacz I, Pardi N, Seliga A, Mui BL, Tam YK, Glassman PM, Shuvaev VV, Nong J, Brenner JS, Khoshnejad M, Madden T, Weissmann D, Persidsky Y, Muzykantov VR, Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood–brain barrier, Proc Natl Acad Sci U S A, 117 (2020) 3405–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Myerson JW, Braender B, McPherson O, Glassman PM, Kiseleva RY, Shuvaev VV, Marcos-Contreras O, Grady ME, Lee H-S, Greineder CF, Stan RV, Composto RJ, Eckmann DM, Muzykantov VR, Flexible Nanoparticles Reach Sterically Obscured Endothelial Targets Inaccessible to Rigid Nanoparticles, Adv Mater (Deerfield Beach, Fla.), 30 (2018) e1802373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Weissleder R, Ntziachristos V, Shedding light onto live molecular targets, Nat Med, 9 (2003) 123128. [DOI] [PubMed] [Google Scholar]
- [217].Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y, Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection, J Biotechnol, 165 (2013) 77–84. [DOI] [PubMed] [Google Scholar]
- [218].Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO, Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter, ACS Nano, 8 (2014) 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Gangadaran P, Li XJ, Lee HW, Oh JM, Kalimuthu S, Rajendran RL, Son SH, Baek SH, Singh TD, Zhu L, Jeong SY, Lee SW, Lee J, Ahn BC, A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice, Oncotarget, 8 (2017) 109894–109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Hikita T, Miyata M, Watanabe R, Oneyama C, Sensitive and rapid quantification of exosomes by fusing luciferase to exosome marker proteins,Sci Rep, 8 (2018) 14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Cashikar AG, Hanson PI, A cell-based assay for CD63-containing extracellular vesicles, PLOS ONE, 14 (2019) e0220007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Parhiz H, Shuvaev VV, Pardi N, Khoshnejad M, Kiseleva RY, Brenner JS, Uhler T, Tuyishime S, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D, Muzykantov VR, PECAM-1 directed retargeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake,J Control Release, 291 (2018) 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D, Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes, J Control Release, 217 (2015) 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Weigert R, Sramkova M, Parente L, Amornphimoltham P, Masedunskas A, Intravital microscopy: a novel tool to study cell biology in living animals, Histochem Cell Bio, 133 (2010) 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Shitara A, Malec L, Ebrahim S, Chen D, Bleck C, Hoffman MP, Weigert R, Cdc42 negatively regulates endocytosis during apical membrane maintenance in live animals, Mol Biol Cell, 30 (2019) 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Schuh CD, Polesel M, Platonova E, Haenni D, Gassama A, Tokonami N, Ghazi S, Bugarski M, Devuyst O, Ziegler U, Hall AM, Combined Structural and Functional Imaging of the Kidney Reveals Major Axial Differences in Proximal Tubule Endocytosis, J Am Soc Nephrol: JASN, 29 (2018) 2696–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Fan W, Xia D, Zhu Q, Li X, He S, Zhu C, Guo S, Hovgaard L, Yang M, Gan Y, Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery, Biomaterials, 151 (2018) 13–23. [DOI] [PubMed] [Google Scholar]
- [228].Grandjean CL, Montalvao F, Celli S, Michonneau D, Breart B, Garcia Z, Perro M, Freytag O, Gerdes CA, Bousso P, Intravital imaging reveals improved Kupffer cell-mediated phagocytosis as a mode of action of glycoengineered anti-CD20 antibodies, Sci Rep, 6 (2016) 34382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Amin M, Mansourian M, Koning GA, Badiee A, Jaafari MR, ten Hagen TLM, Development of a novel cyclic RGD peptide for multiple targeting approaches of liposomes to tumor region, J Control Release, 220 (2015) 308–315. [DOI] [PubMed] [Google Scholar]
- [230].Davidson SM, Jonas O, Keibler MA, Hou HW, Luengo A, Mayers JR, Wyckoff J, Del Rosario AM, Whitman M, Chin CR, Condon KJ, Lammers A, Kellersberger KA, Stall BK, Stephanopoulos G, Bar-Sagi D, Han J, Rabinowitz JD, Cima MJ, Langer R, Vander Heiden MG, Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors, Nat Med, 23 (2017) 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Smith BR, Kempen P, Bouley D, Xu A, Liu Z, Melosh N, Dai H, Sinclair R, Gambhir SS, Shape matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation, Nano lett, 12 (2012) 3369–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Sramkova M, Masedunskas A, Parente L, Molinolo A, Weigert R, Expression of plasmid DNA in the salivary gland epithelium: novel approaches to study dynamic cellular processes in live animals, Am J Physiol. Cell Physiol, 297 (2009) C1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Sramkova M, Masedunskas A, Weigert R, Plasmid DNA is internalized from the apical plasma membrane of the salivary gland epithelium in live animals, Histochem Cell Biol, 138 (2012) 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, Breakefield XO, Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters, Nat Comm, 6 (2015) 7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Joensuu M, Martínez-Mármol R, Padmanabhan P, Glass NR, Durisic N, Pelekanos M, Mollazade M, Balistreri G, Amor R, Cooper-White JJ, Goodhill GJ, Meunier FA, Visualizing endocytic recycling and trafficking in live neurons by subdiffractional tracking of internalized molecules, Nature Protoc, 12 (2017) 2590–2622. [DOI] [PubMed] [Google Scholar]
- [236].van der Zwaag D, Vanparijs N, Wijnands S, De Rycke R, De Geest BG, Albertazzi L, Super Resolution Imaging of Nanoparticles Cellular Uptake and Trafficking, ACS App Mater & Interfaces, 8 (2016) 6391–6399. [DOI] [PubMed] [Google Scholar]
- [237].Masedunskas A, Porat-Shliom N, Rechache K, Aye MP, Weigert R, Intravital Microscopy Reveals Differences in the Kinetics of Endocytic Pathways between Cell Cultures and Live Animals, Cells, 1 (2012) 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Jiang W, Kim BY, Rutka JT, Chan WC, Nanoparticle-mediated cellular response is size-dependent, Nat Nanotechnol, 3 (2008) 145–150. [DOI] [PubMed] [Google Scholar]
- [239].Oh JM, Choi SJ, Lee GE, Kim JE, Choy JH, Inorganic metal hydroxide nanoparticles for targeted cellular uptake through clathrin-mediated endocytosis, Chemistry–An Asian Journal, 4 (2009) 67–73. [DOI] [PubMed] [Google Scholar]
- [240].Suen WLL, Chau Y, Size-dependent internalisation of folate-decorated nanoparticles via the pathways of clathrin and caveolae-mediated endocytosis in ARPE-19 cells, J Pharm Pharmacol, 66 (2014) 564–573. [DOI] [PubMed] [Google Scholar]
- [241].Rejman J, Oberle V, Zuhorn IS, Hoekstra D, Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis,Biochem J, 377 (2004) 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Myerson JW, Braender B, Mcpherson O, Glassman PM, Kiseleva RY, Shuvaev VV, Marcos-Contreras O, Grady ME, Lee HS, Greineder CF, Flexible nanoparticles reach sterically obscured endothelial targets inaccessible to rigid nanoparticles, Adv Mater, 30 (2018) 1802373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Ho YT, Kamm RD, Kah JCY, Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis, Nanoscale, 10 (2018) 12386–12397. [DOI] [PubMed] [Google Scholar]
- [244].Duncan R, Pratten MK, Cable HC, Ringsdorf H, Lloyd JB, Effect of molecular size of 125I-labelled poly(vinylpyrrolidone) on its pinocytosis by rat visceral yolk sacs and rat peritoneal macrophages,Biochem J, 196 (1981) 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH, Size of IgG-opsonized particles determines macrophage response during internalization, Exp Cell Res, 242 (1998) 265–273. [DOI] [PubMed] [Google Scholar]
- [246].Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM, The effect of particle design on cellular internalization pathways, Proc Natl Acad Sci U S A, 105 (2008) 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR, Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells, Blood,J Am Soc Hematol, 99 (2002) 912–922. [DOI] [PubMed] [Google Scholar]
- [248].Shuvaev VV, Muro S, Arguiri E, Khoshnejad M, Tliba S, Christofidou-Solomidou M, Muzykantov VR, Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates, J Control Release, 234 (2016) 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Anselmo AC, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, Mitragotri S, Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting, ACS nano, 9 (2015) 3169–3177. [DOI] [PubMed] [Google Scholar]
- [250].Champion JA, Mitragotri S, Shape induced inhibition of phagocytosis of polymer particles, Pharm Res, 26 (2009) 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Champion JA, Mitragotri S, Role of target geometry in phagocytosis, Proc Natl Acad Sci U S A, 103 (2006) 4930–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S, Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium, Proc Natl Acad Sci U S A, 110 (2013) 10753–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Shimoni O, Yan Y, Wang Y, Caruso F, Shape-dependent cellular processing of polyelectrolyte capsules, ACS nano, 7 (2013) 522–530. [DOI] [PubMed] [Google Scholar]
- [254].Chithrani BD, Chan WC, Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes, Nano lett, 7 (2007) 1542–1550. [DOI] [PubMed] [Google Scholar]
- [255].Cho EC, Au L, Zhang Q, Xia Y, The effects of size, shape, and surface functional group of gold nanostructures on their adsorption and internalization by cells, Small, 6 (2010) 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Hao N, Li L, Zhang Q, Huang X, Meng X, Zhang Y, Chen D, Tang F, Li L, The shape effect of PEGylated mesoporous silica nanoparticles on cellular uptake pathway in Hela cells, Microporous and mesoporous materials, 162 (2012) 14–23. [Google Scholar]
- [257].Seib FP, Jones AT, Duncan R, Comparison of the endocytic properties of linear and branched PEIs, and cationic PAMAM dendrimers in B16f10 melanoma cells, J Control Release, 117 (2007) 291–300. [DOI] [PubMed] [Google Scholar]
- [258].Myerson JW, Anselmo AC, Liu Y, Mitragotri S, Eckmann DM, Muzykantov VR, Non-affinity factors modulating vascular targeting of nano-and microcarriers, Adv Drug Deliv Rev, 99 (2016) 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Donahue ND, Acar H, Wilhelm S, Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine, Adv Drug Deliv Rev, 143 (2019) 68–96. [DOI] [PubMed] [Google Scholar]
- [260].Chou LY, Ming K, Chan WC, Strategies for the intracellular delivery of nanoparticles, Chem Soc Rev, 40 (2011) 233–245. [DOI] [PubMed] [Google Scholar]
- [261].Stewart MP, Lorenz A, Dahlman J, Sahay G, Challenges in carrier-mediated intracellular delivery: moving beyond endosomal barriers, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 8 (2016) 465–478. [DOI] [PubMed] [Google Scholar]
- [262].Frohlich E, Cellular elimination of nanoparticles, Environ Toxicol Pharmacol, 46 (2016) 90–94. [DOI] [PubMed] [Google Scholar]
- [263].Chu Z, Huang Y, Tao Q, Li Q, Cellular uptake, evolution, and excretion of silica nanoparticles in human cells, Nanoscale, 3 (2011) 3291–3299. [DOI] [PubMed] [Google Scholar]
- [264].Oh N, Park JH, Endocytosis and exocytosis of nanoparticles in mammalian cells, Int J Nanomed, 9 Suppl 1 (2014) 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Sakhtianchi R, Minchin RF, Lee KB, Alkilany AM, Serpooshan V, Mahmoudi M, Exocytosis of nanoparticles from cells: role in cellular retention and toxicity, Adv Colloid Interface Sci, 201–202 (2013) 18–29. [DOI] [PubMed] [Google Scholar]
- [266].Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, Langer R, Farokhzad OC, Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery, Sci Transl Med, 5 (2013) 213ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Ferkol T, Cohn LA, Phillips TE, Smith A, Davis PB, Targeted delivery of antiprotease to the epithelial surface of human tracheal xenografts, Am J Respir Crit Care Med, 167 (2003) 1374–1379. [DOI] [PubMed] [Google Scholar]
- [268].Eckman EA, Mallender WD, Szegletes T, Silski CL, Schreiber JR, Davis PB, Ferkol TW, In vitro transport of active alpha(1)-antitrypsin to the apical surface of epithelia by targeting the polymeric immunoglobulin receptor, Am J Respir Cell Mol Biol, 21 (1999) 246–252. [DOI] [PubMed] [Google Scholar]
- [269].Pardridge WM, Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody, Expert Opin Drug Deliv, 12 (2015) 207–222. [DOI] [PubMed] [Google Scholar]
- [270].Pandit R, Chen L, Gotz J, The blood-brain barrier: Physiology and strategies for drug delivery, Adv Drug Deliv Rev, (2019). [DOI] [PubMed] [Google Scholar]
- [271].Banks WA, From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery, Nat Rev Drug Discov, 15 (2016) 275–292. [DOI] [PubMed] [Google Scholar]
- [272].Liu X, Jiang J, Meng H, Transcytosis - An effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery, Theranostics, 9 (2019) 8018–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Hsueh PY, Edman MC, Sun G, Shi P, Xu S, Lin YA, Cui H, Hamm-Alvarez SF, MacKay JA, Tear-mediated delivery of nanoparticles through transcytosis of the lacrimal gland,J Control Release, 208 (2015) 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Duncan R, Designing polymer conjugates as lysosomotropic nanomedicines, Biochem Soc Transact, 35 (2007) 56–60. [DOI] [PubMed] [Google Scholar]
- [275].Nair LS, Laurencin CT, Biodegradable polymers as biomaterials, Prog Polym Sci, 32 (2007) 762–798. [Google Scholar]
- [276].Gruessner U, Clemens M, Pahlplatz PV, Sperling P, Witte J, Rosen HR, Group SS, Improvement of perineal wound healing by local administration of gentamicin-impregnated collagen fleeces after abdominoperineal excision of rectal cancer, Am J Surg, 182 (2001) 502–509. [DOI] [PubMed] [Google Scholar]
- [277].Sano A, Maeda M, Nagahara S, Ochiya T, Honma K, Itoh H, Miyata T, Fujioka K, Atelocollagen for protein and gene delivery, Adv Drug Deliv Rev, 55 (2003) 1651–1677. [DOI] [PubMed] [Google Scholar]
- [278].Mithieux SM, Rasko JE, Weiss AS, Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers, Biomaterials, 25 (2004) 4921–4927. [DOI] [PubMed] [Google Scholar]
- [279].Kishida A, Murakami K, Goto H, Akashi M, Kubota H, Endo T, Polymer drugs and polymeric drugs X: slow release of 5-fluorouracil from biodegradable poly (γ-glutamic acid) and its benzyl ester matrices, J Bioact Compat Pol, 13 (1998) 270–278. [Google Scholar]
- [280].Nicol F, Wong M, MacLaughlin F, Perrard J, Wilson E, Nordstrom J, Smith L, Poly-L-glutamate, an anionic polymer, enhances transgene expression for plasmids delivered by intramuscular injection with in vivo electroporation, Gene Ther, 9 (2002) 1351–1358. [DOI] [PubMed] [Google Scholar]
- [281].Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y, Shirao K, Okusaka T, Ueno H, Ikeda M, Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin, Bri J Cancer, 91 (2004) 1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Shah M, Hsueh PY, Sun G, Chang HY, Janib SM, MacKay JA, Biodegradation of elastin-like polypeptide nanoparticles, Prot Sci, 21 (2012) 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Guo H, Lee C, Shah M, Janga SR, Edman MC, Klinngam W, Hamm-Alvarez SF, MacKay JA, A novel elastin-like polypeptide drug carrier for cyclosporine A improves tear flow in a mouse model of Sjögren’s syndrome, J Control Release, 292 (2018) 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Li Z, Sreekumar PG, Peddi S, Hinton DR, Kannan R, MacKay JA, The humanin peptide mediates ELP nanoassembly and protects human retinal pigment epithelial cells from oxidative stress,Nanomed-Nanotechnol, 24 (2020) 102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Hsueh P-Y, Ju Y, Vega A, Edman MC, MacKay JA, Hamm-Alvarez SF, A Multivalent ICAM-1 Binding Nanoparticle which Inhibits ICAM-1 and LFA-1 Interaction Represents a New Tool for the Investigation of Autoimmune-Mediated Dry Eye, Int J Mol Sci, 21 (2020) 2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Luo Y, Kirker KR, Prestwich GD, Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery, J Control Release, 69 (2000) 169–184. [DOI] [PubMed] [Google Scholar]
- [287].Bhattarai N, Gunn J, Zhang M, Chitosan-based hydrogels for controlled, localized drug delivery, Adv Drug Deliv Rev, 62 (2010) 83–99. [DOI] [PubMed] [Google Scholar]
- [288].Muro S, Strategies for delivery of therapeutics into the central nervous system for treatment of lysosomal storage disorders, Drug Deliv Transl Res, 2 (2012) 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].LeBowitz JH, Grubb JH, Maga JA, Schmiel DH, Vogler C, Sly WS, Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice, Proc Natl Acad Sci U S A, 101 (2004) 3083–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Prince WS, McCormick LM, Wendt DJ, Fitzpatrick PA, Schwartz KL, Aguilera AI, Koppaka V, Christianson TM, Vellard MC, Pavloff N, Lipoprotein receptor binding, cellular uptake, and lysosomal delivery of fusions between the receptor-associated protein (RAP) and α-L-iduronidase or acid α-glucosidase, J Bio Chem, 279 (2004) 35037–35046. [DOI] [PubMed] [Google Scholar]
- [291].Garnacho C, Dhami R, Solomon M, Schuchman EH, Muro S, Enhanced Delivery and Effects of Acid Sphingomyelinase by ICAM-1-Targeted Nanocarriers in Type B Niemann-Pick Disease Mice, Mol Ther, 25 (2017) 1686–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Garnacho C, Muro S, ICAM-1 targeting, intracellular trafficking, and functional activity of polymer nanocarriers coated with a fibrinogen-derived peptide for lysosomal enzyme replacement,J Drug Target, 25 (2017) 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Hsu J, Hoenicka J, Muro S, Targeting, Endocytosis, and Lysosomal Delivery of Active Enzymes to Model Human Neurons by ICAM-1-Targeted Nanocarriers, Pharmaceutical Research, 32 (2015) 1264–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Cabrera I, Abasolo I, Corchero JL, Elizondo E, Gil PR, Moreno E, Faraudo J, Sala S, Bueno D, González-Mira E, Rivas M, Melgarejo M, Pulido D, Albericio F, Royo M, Villaverde A, García-Parajo MF, Schwartz S Jr., Ventosa N, Veciana J, α-Galactosidase-A Loaded-Nanoliposomes with Enhanced Enzymatic Activity and Intracellular Penetration, Adv Healthc Mater, 5 (2016) 829–840. [DOI] [PubMed] [Google Scholar]
- [295].Giannotti MI, Abasolo I, Oliva M, Andrade F, Garcia-Aranda N, Melgarejo M, Pulido D, Corchero JL, Fernandez Y, Villaverde A, Royo M, Garcia-Parajo MF, Sanz F, Schwartz S Jr., Highly Versatile Polyelectrolyte Complexes for Improving the Enzyme Replacement Therapy of Lysosomal Storage Disorders, ACS Appl Mater Interfaces, 8 (2016) 25741–25752. [DOI] [PubMed] [Google Scholar]
- [296].Moreno-Layseca P, Icha J, Hamidi H, Ivaska J, Integrin trafficking in cells and tissues, Nat Cell Bio, 21 (2019) 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Simons M, Gordon E, Claesson-Welsh L, Mechanisms and regulation of endothelial VEGF receptor signalling, Nat Rev Mol Cell Bio, 17 (2016) 611–625. [DOI] [PubMed] [Google Scholar]
- [298].Storch J, Cheruku SR, Cholesterol Transport in Lysosomes, in: Saftig P (Ed.) Lysosomes, Springer US, Boston, MA, 2005, pp. 100–111. [Google Scholar]
- [299].Fu Y, Jussi J, Wang Q, Brismar H, Liu Y, Yang X, Chen Y, Endocytic pathway of vascular cell adhesion molecule 1 in human umbilical vein endothelial cell identified in vitro by using functionalized nontoxic fluorescent quantum dots, Sensors and Actuators B: Chemical, 297 (2019) 126702. [Google Scholar]
- [300].Tomas A, Futter CE, Eden ER, EGF receptor trafficking: consequences for signaling and cancer, Trends Cell Biol, 24 (2014) 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Varkouhi AK, Scholte M, Storm G, Haisma HJ, Endosomal escape pathways for delivery of biologicals, J Control Release, 151 (2011) 220–228. [DOI] [PubMed] [Google Scholar]
- [302].Shete HK, Prabhu RH, Patravale VB, Endosomal escape: a bottleneck in intracellular delivery, J Nanosci Nanotechnol, 14 (2014) 460–474. [DOI] [PubMed] [Google Scholar]
- [303].Stegmann T, Membrane fusion mechanisms: the influenza hemagglutinin paradigm and its implications for intracellular fusion, Traffic (Copenhagen, Denmark), 1 (2000) 598–604. [DOI] [PubMed] [Google Scholar]
- [304].Lear JD, DeGrado WF, Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2, J Bio Chem, 262 (1987) 6500–6505. [PubMed] [Google Scholar]
- [305].Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E, The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems, J Bio Chem, 269 (1994) 12918–12924. [PubMed] [Google Scholar]
- [306].Subramanian A, Ma H, Dahl KN, Zhu J, Diamond SL, Adenovirus or HA-2 fusogenic peptide-assisted lipofection increases cytoplasmic levels of plasmid in nondividing endothelium with little enhancement of transgene expression,J Gene Med, 4 (2002) 75–83. [DOI] [PubMed] [Google Scholar]
- [307].Liou J-S, Liu BR, Martin AL, Huang Y-W, Chiang H-J, Lee H-J, Protein transduction in human cells is enhanced by cell-penetrating peptides fused with an endosomolytic HA2 sequence, Peptides, 37 (2012) 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Oliveira S, van Rooy I, Kranenburg O, Storm G, Schiffelers RM, Fusogenic peptides enhance endosomal escape improving siRNA-induced silencing of oncogenes, Int J Pharm, 331 (2007) 211–214. [DOI] [PubMed] [Google Scholar]
- [309].Mastrobattista E, Koning GA, van Bloois L, Filipe AC, Jiskoot W, Storm G, Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins, J Bio Chem, 277 (2002) 27135–27143. [DOI] [PubMed] [Google Scholar]
- [310].Raemdonck K, Naeye B, Buyens K, Vandenbroucke RE, Høgset A, Demeester J, De Smedt SC, Biodegradable dextran nanogels for RNA interference: focusing on endosomal escape and intracellular siRNA delivery, Adv. Funct. Mater, 19 (2009) 1406–1415. [Google Scholar]
- [311].Simeoni F, Morris MC, Heitz F, Divita G, Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells, Nucleic Acids Res, 31 (2003) 2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Chernomordik LV, Melikyan GB, Chizmadzhev YA, Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers, Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes, 906 (1987) 309–352. [DOI] [PubMed] [Google Scholar]
- [313].Lorenzi GL, Lee KD, Enhanced plasmid DNA delivery using anionic LPDII by listeriolysin O incorporation,J Gene Med, 7 (2005) 1077–1085. [DOI] [PubMed] [Google Scholar]
- [314].Kullberg M, Owens JL, Mann K, Listeriolysin O enhances cytoplasmic delivery by Her-2 targeting liposomes,J Drug Target, 18 (2010) 313–320. [DOI] [PubMed] [Google Scholar]
- [315].Walton CM, Wu CH, Wu GY, A DNA delivery system containing listeriolysin O results in enhanced hepatocyte-directed gene expression, World J Gastroenterol, 5 (1999) 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Saito G, Amidon G, Lee K, Enhanced cytosolic delivery of plasmid DNA by a sulfhydryl-activatable listeriolysin O/protamine conjugate utilizing cellular reducing potential, Gene Ther, 10 (2003) 72–83. [DOI] [PubMed] [Google Scholar]
- [317].Kakimoto S, Hamada T, Komatsu Y, Takagi M, Tanabe T, Azuma H, Shinkai S, Nagasaki T, The conjugation of diphtheria toxin T domain to poly (ethylenimine) based vectors for enhanced endosomal escape during gene transfection, Biomaterials, 30 (2009) 402–408. [DOI] [PubMed] [Google Scholar]
- [318].Barati S, Chegini F, Hurtado P, Rush RA, Hybrid tetanus toxin C fragment-diphtheria toxin translocation domain allows specific gene transfer into PC12 cells, Exp Neurol, 177 (2002) 75–87. [DOI] [PubMed] [Google Scholar]
- [319].Rasper DM, Merrill AR, Evidence for the modulation of Pseudomonas aeruginosa exotoxin A-induced pore formation by membrane surface charge density, Biochemistry, 33 (1994) 12981–12989. [DOI] [PubMed] [Google Scholar]
- [320].Teter K, Holmes RK, Inhibition of endoplasmic reticulum-associated degradation in CHO cells resistant to cholera toxin, Pseudomonas aeruginosa exotoxin A, and ricin,Infect Immun, 70 (2002) 6172–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Jia L-T, Zhang L-H, Yu C-J, Zhao J, Xu Y-M, Gui J-H, Jin M, Ji Z-L, Wen W-H, Wang C-J, Specific tumoricidal activity of a secreted proapoptotic protein consisting of HER2 antibody and constitutively active caspase-3, Cancer Res, 63 (2003) 3257–3262. [PubMed] [Google Scholar]
- [322].Bruell D, Stöcker M, Huhn M, Redding N, Küpper M, Schumacher P, Paetz A, Bruns CJ, Haisma HJ, Fischer R, The recombinant anti-EGF receptor immunotoxin 425 (scFv)-ETA’suppresses growth of a highly metastatic pancreatic carcinoma cell line, Int J Oncol, 23 (2003) 1179–1186. [DOI] [PubMed] [Google Scholar]
- [323].Prior TI, FitzGerald DJ, Pastan I, Translocation mediated by domain II of Pseudomonas exotoxin A: transport of barnase into the cytosol, Biochemistry, 31 (1992) 3555–3559. [DOI] [PubMed] [Google Scholar]
- [324].Min SH, Lee DC, Lim MJ, Park HS, Kim DM, Cho CW, Yoon DY, Yeom YI, A composite gene delivery system consisting of polyethylenimine and an amphipathic peptide KALA,J Gene Med, 8 (2006) 1425–1434. [DOI] [PubMed] [Google Scholar]
- [325].Lee H, Jeong JH, Park TG, A new gene delivery formulation of polyethylenimine/DNA complexes coated with PEG conjugated fusogenic peptide, J Control Release, 76 (2001) 183–192. [DOI] [PubMed] [Google Scholar]
- [326].Han J, Yeom YI, Specific gene transfer mediated by galactosylated poly-L-lysine into hepatoma cells, Int J Pharm, 202 (2000) 151–160. [DOI] [PubMed] [Google Scholar]
- [327].Futaki S, Masui Y, Nakase I, Sugiura Y, Nakamura T, Kogure K, Harashima H, Unique features of a pH-sensitive fusogenic peptide that improves the transfection efficiency of cationic liposomes,J Gene Med, 7 (2005) 1450–1458. [DOI] [PubMed] [Google Scholar]
- [328].Sasaki K, Kogure K, Chaki S, Nakamura Y, Moriguchi R, Hamada H, Danev R, Nagayama K, Futaki S, Harashima H, An artificial virus-like nano carrier system: enhanced endosomal escape of nanoparticles via synergistic action of pH-sensitive fusogenic peptide derivatives, Anal Bioanal Chem, 391 (2008) 2717–2727. [DOI] [PubMed] [Google Scholar]
- [329].Kakudo T, Chaki S, Futaki S, Nakase I, Akaji K, Kawakami T, Maruyama K, Kamiya H, Harashima H, Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: an artificial viral-like delivery system, Biochemistry, 43 (2004) 5618–5628. [DOI] [PubMed] [Google Scholar]
- [330].Simoes S, Slepushkin V, Pires P, Gaspar R, de Lima MP, Düzgüneş N, Mechanisms of gene transfer mediated by lipoplexes associated with targeting ligands or pH-sensitive peptides, Gene Ther, 6 (1999) 1798–1807. [DOI] [PubMed] [Google Scholar]
- [331].Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr J-P, A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine, Proc Natl Acad Sci U S A, 92 (1995) 7297–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Kim T-H, Choi H, Yu GS, Choi JS, Synthesis of polyethylene glycol-oligo (glutamic acid) conjugated with polyethylenimine-dexamethasone for gene delivery applications, J Nanosci Nanotechnol, 13 (2013) 7325–7330. [DOI] [PubMed] [Google Scholar]
- [333].Haensler J, Szoka FC Jr, Polyamidoamine cascade polymers mediate efficient transfection of cells in culture, Bioconj Chem, 4 (1993) 372–379. [DOI] [PubMed] [Google Scholar]
- [334].Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, Minko T, Discher DE, Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation, Mol Pharm, 3 (2006) 340–350. [DOI] [PubMed] [Google Scholar]
- [335].Zelmer C, Zweifel LP, Kapinos LE, Craciun I, Güven ZP, Palivan CG, Lim RYH, Organelle-specific targeting of polymersomes into the cell nucleus, Proc Natl Acad Sci U S A, 117 (2020) 2770–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Kang B, Mackey MA, El-Sayed MA, Nuclear Targeting of Gold Nanoparticles in Cancer Cells Induces DNA Damage, Causing Cytokinesis Arrest and Apoptosis,J Am Chem Soc, 132 (2010) 1517–1519. [DOI] [PubMed] [Google Scholar]
- [337].Tammam SN, Azzazy HME, Lamprecht A, The effect of nanoparticle size and NLS density on nuclear targeting in cancer and normal cells; impaired nuclear import and aberrant nanoparticle intracellular trafficking in glioma, J Control Release, 253 (2017) 30–36. [DOI] [PubMed] [Google Scholar]
- [338].Zanta MA, Belguise-Valladier P, Behr J-P, Gene delivery: A single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus, Proc Natl Acad Sci U S A, 96 (1999) 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Chen K, Guo L, Zhang J, Chen Q, Wang K, Li C, Li W, Qiao M, Zhao X, Hu H, Chen D, A gene delivery system containing nuclear localization signal: Increased nucleus import and transfection efficiency with the assistance of RanGAP1, Acta Biomater, 48 (2017) 215–226. [DOI] [PubMed] [Google Scholar]
- [340].Boshnjaku V, Shim KW, Tsurubuchi T, Ichi S, Szany EV, Xi G, Mania-Farnell B, McLone DG, Tomita T, Mayanil CS, Nuclear localization of folate receptor alpha: a new role as a transcription factor, Sci Rep, 2 (2012) 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Jin X, Zhang J, Jin X, Liu L, Tian X, Folate Receptor Targeting and Cathepsin B-Sensitive Drug Delivery System for Selective Cancer Cell Death and Imaging, ACS Med Chem Lett, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Wang Y, Zhang C, Li H, Zhu G, Bao S-S, Wei S, Zheng L-M, Ren M, Xu Z, Synthesis, characterization and in vitro anticancer activity of the biomolecule-based coordination complex nanotubes, J Mater Chem B, 3 (2015) 296–305. [DOI] [PubMed] [Google Scholar]
- [343].Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP, Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay, Anal Biochem, 338 (2005) 284–293. [DOI] [PubMed] [Google Scholar]
- [344].Scaranti M, Cojocaru E, Banerjee S, Banerji U, Exploiting the folate receptor α in oncology, Nat Rev Clin Oncol, 17 (2020) 349–359. [DOI] [PubMed] [Google Scholar]
- [345].Müller C, Schibli R, Prospects in folate receptor-targeted radionuclide therapy, Front Oncol, 3 (2013) 249-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Fernández M, Javaid F, Chudasama V, Advances in targeting the folate receptor in the treatment/imaging of cancers, Chem Sci, 9 (2018) 790–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Sabharanjak S, Mayor S, Folate receptor endocytosis and trafficking, Adv Drug Deliv Rev, 56 (2004) 1099–1109. [DOI] [PubMed] [Google Scholar]
- [348].Mukhopadhyay A, Ni L, Yang CS, Weiner H, Bacterial signal peptide recognizes HeLa cell mitochondrial import receptors and functions as a mitochondrial leader sequence, Cell Mol Life Sci, 62 (2005) 1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Murphy MP, Smith RA, Drug delivery to mitochondria: the key to mitochondrial medicine, Adv Drug Deliv Rev, 41 (2000) 235–250. [DOI] [PubMed] [Google Scholar]
- [350].Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, Yamashita K, Kobayashi H, Kikuchi H, Harashima H, MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion, Biochim Biophys Acta, 1778 (2008) 423–432. [DOI] [PubMed] [Google Scholar]
- [351].Yamada Y, Furukawa R, Yasuzaki Y, Harashima H, Dual function MITO-Porter, a nano carrier integrating both efficient cytoplasmic delivery and mitochondrial macromolecule delivery, Mol Ther, 19 (2011) 1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Milane L, Trivedi M, Singh A, Talekar M, Amiji M, Mitochondrial biology, targets, and drug delivery,J Control Release, 207 (2015) 40–58. [DOI] [PubMed] [Google Scholar]
- [353].Yang Y, Karakhanova S, Hartwig W, D’Haese JG, Philippov PP, Werner J, Bazhin AV, Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy, J Cell Physiol, 231 (2016) 2570–2581. [DOI] [PubMed] [Google Scholar]
- [354].Lin JH, Walter P, Yen TSB, Endoplasmic reticulum stress in disease pathogenesis, Annu Rev Pathol, 3 (2008) 399–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Wang G, Norton AS, Pokharel D, Song Y, Hill RA, KDEL peptide gold nanoconstructs: promising nanoplatforms for drug delivery,Nanomed-Nanotechnol, 9 (2013) 366–374. [DOI] [PubMed] [Google Scholar]
- [356].Ghosh C, Nandi A, Basu S, Lipid Nanoparticle-Mediated Induction of Endoplasmic Reticulum Stress in Cancer Cells, ACS Applied Bio Materials, 2 (2019) 3992–4001. [DOI] [PubMed] [Google Scholar]
- [357].Deng H, Zhou Z, Yang W, Lin L.-s., Wang S, Niu G, Song J, Chen X, Endoplasmic Reticulum Targeting to Amplify Immunogenic Cell Death for Cancer Immunotherapy, Nano lett, 20 (2020) 1928–1933. [DOI] [PubMed] [Google Scholar]
- [358].Sneh-Edri H, Likhtenshtein D, Stepensky D, Intracellular Targeting of PLGA Nanoparticles Encapsulating Antigenic Peptide to the Endoplasmic Reticulum of Dendritic Cells and Its Effect on Antigen Cross-Presentation in Vitro, Mol Pharm, 8 (2011) 1266–1275. [DOI] [PubMed] [Google Scholar]
- [359].Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H, Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis, J Exp Med, 174 (1991) 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Leifert JA, Rodriguez-Carreno MP, Rodriguez F, Lindsay Whitton J, Targeting plasmid-encoded proteins to the antigen presentation pathways, Immunol Rev, 199 (2004) 40–53. [DOI] [PubMed] [Google Scholar]
- [361].Sher YP, Lin SI, Chai KM, Chen IH, Liu SJ, Endoplasmic reticulum-targeting sequence enhanced the cellular immunity of a tumor-associated antigen L6-based DNA vaccine, Am J Cancer Res, 9 (2019) 2028–2036. [PMC free article] [PubMed] [Google Scholar]
- [362].Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS, Expression profiling reveals off-target gene regulation by RNAi, Nat Biotechnol, 21 (2003) 635–637. [DOI] [PubMed] [Google Scholar]
- [363].Hinrichsen L, Harborth J, Andrees L, Weber K, Ungewickell EJ, Effect of clathrin heavy chain- and α-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells, J Bio Chem, 278 (2003) 45160–45170. [DOI] [PubMed] [Google Scholar]
- [364].Richardson SC, Wallom KL, Ferguson EL, Deacon SP, Davies MW, Powell AJ, Piper RC, Duncan R, The use of fluorescence microscopy to define polymer localisation to the late endocytic compartments in cells that are targets for drug delivery,J Control Release, 127 (2008) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA, Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes, PLOS Pathogens, 6 (2010) e1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Chen L, Cheng D, Chu J, Zhang T, Dong Z, Lou H, Zhu L, Liu Y, A Novel Method to Image Macropinocytosis in Vivo, Front Neurosci, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Fernando LP, Kandel PK, Yu J, McNeill J, Ackroyd PC, Christensen KA, Mechanism of cellular uptake of highly fluorescent conjugated polymer nanoparticles, Biomacromolecules, 11 (2010) 2675–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Zenni MK, Giardina PC, Harvey HA, Shao J, Ketterer MR, Lubaroff DM, Williams RD, Apicella MA, Macropinocytosis as a mechanism of entry into primary human urethral epithelial cells by Neisseria gonorrhoeae,Infect Immun, 68 (2000) 1696–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Choi CHJ, Hao L, Narayan SP, Auyeung E, Mirkin CA, Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates, Proc Natl Acad Sci U S A, 110 (2013) 7625–7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Dunn KW, Kamocka MM, McDonald JH, A practical guide to evaluating colocalization in biological microscopy, Am J Physiol-Cell Physiol, 300 (2011) C723–C742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Lencer WI, Hirst TR, Holmes RK, Membrane traffic and the cellular uptake of cholera toxin, Biochimica et Biophysica Acta (BBA) - Mol Cell Res, 1450 (1999) 177–190. [DOI] [PubMed] [Google Scholar]
- [372].Torgersen ML, Skretting G, van Deurs B, Sandvig K, Internalization of cholera toxin by different endocytic mechanisms, J Cell Sci, 114 (2001) 3737–3747. [DOI] [PubMed] [Google Scholar]
- [373].Qaddoumi MG, Gukasyan HJ, Davda J, Labhasetwar V, Kim KJ, Lee VH, Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: role in PLGA nanoparticle endocytosis, Mol Vision, 9 (2003) 559–568. [PubMed] [Google Scholar]
- [374].Oh JM, Choi SJ, Kim ST, Choy JH, Cellular uptake mechanism of an inorganic nanovehicle and its drug conjugates: Enhanced efficacy due to clathrin-mediated endocytosis, Bioconjug Chem, 17 (2006) 1411–1417. [DOI] [PubMed] [Google Scholar]
- [375].Manunta M, Izzo L, Duncan R, Jones AT, Establishment of subcellular fractionation techniques to monitor the intracellular fate of polymer therapeutics II. Identification of endosomal and lysosomal compartments in HepG2 cells combining single-step subcellular fractionation with fluorescent imaging, J Drug Target, 15 (2007) 37–50. [DOI] [PubMed] [Google Scholar]
- [376].Seib FP, Jones AT, Duncan R, Establishment of subcellular fractionation techniques to monitor the intracellular fate of polymer therapeutics I. Differential centrifugation fractionation B16F10 cells and use to study the intracellular fate of HPMA copolymer - doxorubicin, J Drug Target, 14 (2006) 375–390. [DOI] [PubMed] [Google Scholar]
- [377].Yue Y, Behra R, Sigg L, Suter MJF, Pillai S, Schirmer K, Silver nanoparticle–protein interactions in intact rainbow trout gill cells, Environmental Science: Nano, 3 (2016) 1174–1185. [Google Scholar]
- [378].Suárez VT, Karepina E, Chevallet M, Gallet B, Cottet-Rousselle C, Charbonnier P, Moriscot C, Michaud-Soret I, Bal W, Fuchs A, Tucoulou R, Jouneau P-H, Veronesi G, Deniaud A, Visualization of silver nanoparticle intracellular trafficking revealed nuclear translocation of silver ions leading to nuclear receptor impairment, bioRxiv, (2019) 825919. [Google Scholar]
- [379].Shibata A, McMullen E, Pham A, Belshan M, Sanford B, Zhou Y, Goede M, Date AA, Destache CJ, Polymeric Nanoparticles Containing Combination Antiretroviral Drugs for HIV Type 1 Treatment,AIDS Res Hum Retrov, 29 (2013) 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Fanizza E, Depalo N, Fedorenko S, Iacobazzi RM, Mukhametshina A, Zairov R, Salatino A, Vischio F, Panniello A, Laquintana V, Curri ML, Mustafina A, Denora N, Striccoli M, Green Fluorescent Terbium (III) Complex Doped Silica Nanoparticles, Int J Mol Sci, 20 (2019) 3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Jin W, Al-Dulaymi M, Badea I, Leary SC, Rehman J, El-Aneed A, Cellular Uptake and Distribution of Gemini Surfactant Nanoparticles Used as Gene Delivery Agents, The AAPS journal, 21 (2019) 98. [DOI] [PubMed] [Google Scholar]
- [382].Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, Perucco E, Collesi C, Min W, Zeiss C, De Camilli P, Cremona O, Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice, Proc Natl Acad Sci U S A, 106 (2009) 13838–13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Lin HP, Singla B, Ghoshal P, Faulkner JL, Cherian-Shaw M, O’Connor PM, She JX, Belin de Chantemele EJ, Csányi G, Identification of novel macropinocytosis inhibitors using a rational screen of Food and Drug Administration-approved drugs, Br J Pharmacol, 175 (2018) 3640–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Shevtsov MA, Nikolaev BP, Yakovleva LY, Marchenko YY, Dobrodumov AV, Mikhrina AL, Martynova MG, Bystrova OA, Yakovenko IV, Ischenko AM, Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors, Int J Nanomed, 9 (2014) 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Corbin IR, Chen J, Li H, Cao W, Zheng GEDLK, Ntziachristos V, Functionalizing Low-density Lipoprotein Nanoparticles for In Vivo Near-Infrared Optical Imaging of Cancer, Molecular Imaging, Optical Society of America, Munich, 2007, pp. 6626–6610. [Google Scholar]
- [386].Jin H, Lovell JF, Chen J, Lin Q, Ding L, Ng KK, Pandey RK, Manoharan M, Zhang Z, Zheng G, Mechanistic insights into LDL nanoparticle-mediated siRNA delivery, Bioconjug Chem, 23 (2012) 33–41. [DOI] [PubMed] [Google Scholar]
- [387].Vidal M, Sainte-Marie J, Philippot JR, Bienvenue A, LDL-mediated targeting of liposomes to leukemic lymphocytes in vitro, EMBO J, 4 (1985) 2461–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Hild W, Pollinger K, Caporale A, Cabrele C, Keller M, Pluym N, Buschauer A, Rachel R, Tessmar J, Breunig M, Goepferich A, G protein-coupled receptors function as logic gates for nanoparticle binding and cell uptake, Proc Natl Acad Sci U S A, 107 (2010) 10667–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Hörsch D, Ezziddin S, Haug A, Gratz KF, Dunkelmann S, Miederer M, Schreckenberger M, Krause BJ, Bengel FM, Bartenstein P, Biersack HJ, Pöpperl G, Baum RP, Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up, Eur J Cancer (Oxford, England : 1990), 58 (2016) 41–51. [DOI] [PubMed] [Google Scholar]
- [390].Arosio D, Casagrande C, Advancement in integrin facilitated drug delivery, Adv Drug Deliv Rev, 97 (2016) 111–143. [DOI] [PubMed] [Google Scholar]
- [391].Muzykantov VR, Targeted Drug Delivery to Endothelial Adhesion Molecules, ISRN Vascular Medicine, 2013 (2013) 916254. [Google Scholar]