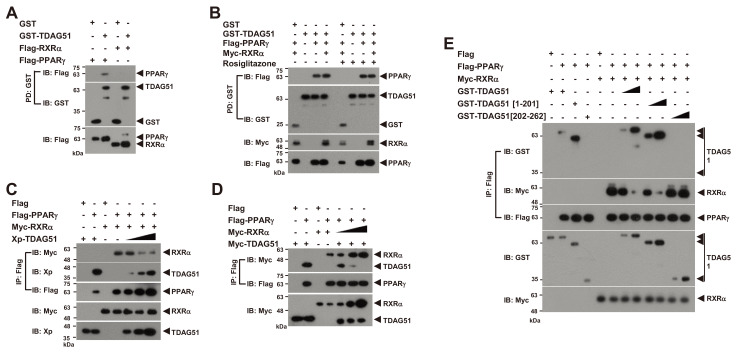
Fig. 4. The interaction of TDAG51 and PPARγ inhibits the formation of the PPARγ-RXRα heterodimer.
(A) No interaction between TDAG51 and RXRα. First, 293T cells were cotransfected with GST-TDAG51 (1.5 µg) with or without Flag-PPARγ (1.5 µg) or Flag-RXRα (0.1 µg). The bound proteins by GST-PD were visualized via anti-Flag (top) or anti-GST IB (middle). The PPARγ and RXRα expression levels in whole cell lysates were detected via anti-Flag IB (bottom). GST alone (mock) was used as a control. Protein expression is indicated by an arrow. (B) No effect of rosiglitazone treatment on the TDAG51-PPARγ interaction. The 293T cells cotransfected with epitope-tagged expression plasmids were treated with 5 μM rosiglitazone for 36 h. The protein interaction was monitored by GST-PD and IB analysis. (C) The inhibitory effect of TDAG51 on the formation of PPARγ-RXRα heterodimers. The Xp-tagged TDAG51 plasmid (Xp-TDAG51 [0.2, 0.5, and 2.0 µg]) was dose-dependently cotransfected with Flag-PPARγ (1.5 µg) and Myc-RXRα (0.1 µg) expression plasmids into 293T cells. The protein interactions were monitored by anti-Flag IP and IB analysis (top three panels). The TDAG51 and RXRα expression levels in whole cell lysates were detected via anti-Xp and anti-Myc IB, respectively (bottom two panels). (D) The inhibitory effect of RXRα on the interaction of PPARγ and TDAG51. The Myc-tagged RXRα plasmid (Myc-RXRα [0.25, 0.5, and 1.0 µg]) was dose-dependently cotransfected with Flag-PPARγ (1.5 µg) and Myc-TDAG51 (1.5 µg) expression plasmids into 293T cells. (E) The inhibitory effects of TDAG51 deletion mutants on the formation of PPARγ-RXRα heterodimers. The GST-tagged TDAG51 deletion mutant plasmids (0.5 and 2.0 µg) were dose-dependently cotransfected with Flag-PPARγ (1.5 µg) and Myc-RXRα (0.1 µg) expression plasmids into 293T cells.