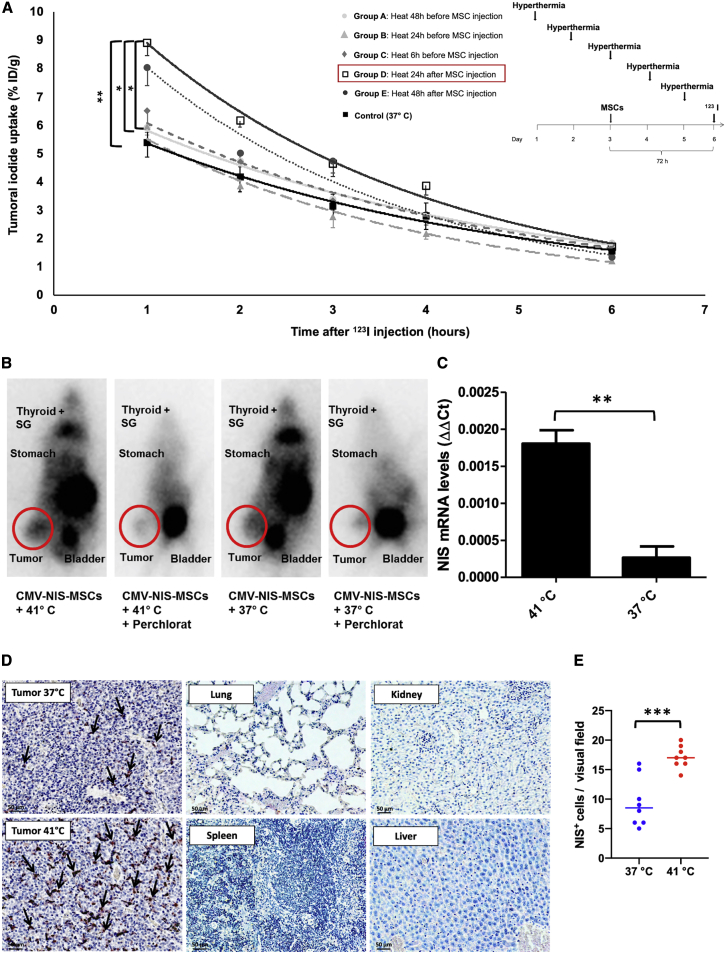
Figure 4.
123I-Scintigraphy following CMV-NIS-MSC Administration
s.c. HuH7 tumor-bearing mice were injected with CMV-NIS-MSCs and subjected to hyperthermic treatment (1 h at 41°C or 37°C, as controls; n = 6) at different time points: 48 h (group A, n = 6), 24 h (group B, n = 6), 6 h (group C, n = 7) prior and 24 h (group D, n = 6, ∗∗p = 0.0055 [group D versus control], ∗p = 0.024 [group D versus group A], ∗p = 0.028 [group D versus group B], p = 0.086 [group D versus group C], p = 0.90 [group D versus group E]) and 48 h (group E, n = 6) after hyperthermia. Three days later, 123I-scintigraphy was performed and tumoral iodine uptake and efflux were analyzed (A). Results are expressed as mean ± SEM, and significance was tested by ANOVA followed by post hoc Tukey (honestly significant difference) test. One representative image for the best performing hyperthermia treatment group and the control group, displaying besides the tumoral iodine accumulation a 123I signal from the endogenously NIS expressing organs, thyroid, SGs, stomach, and the urinary bladder due to 123I elimination. The competitive NIS inhibitor perchlorate was added 30 min prior to 123I administration (B). mRNA isolated from frozen tumor sections was analyzed for NIS by real-time PCR (C) (n = 3; two-tailed Student’s t test; ∗∗p = 0.0033). On paraffin-embedded tumor section, NIS-specific immunohistochemistry (red) was performed on tumors of 37°C control animals, heat-treated tumors of mice, and control organs. One representative image is shown each at 20× magnification, scale bar, 50 μm (D). Quantification of NIS staining on tumor sections (dots represent counts in a single visual field, lines represent the median) (E).