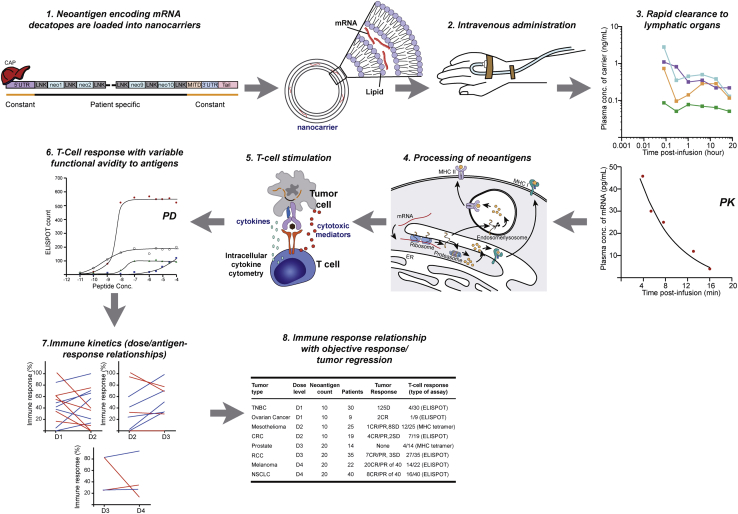
Figure 3.
Complexity of Dosing Strategy and Dose-Response for a Personalized Neoantigen-Based TCV
Favorable absorption and distribution of antigen-encoder/antigen to lymphoid organs are precursors to enable successful immune activation, anti-tumor CTL activity, and effective tumor killing. After neoantigen encoding mRNA is packaged into nanocarriers and infused (steps 1 and 2), mRNA and carrier component concentrations are measured in systemic circulation, which may relate to uptake by lymphoid organs for processing of neoantigens (steps 3 and 4). After processing of neoantigens, immune monitoring of antigen-specific T cell responses is evaluated (step 5); however, due to heterogeneity in dose-response relationships across multiple antigens or epitopes in a personalized TCV (steps 6 and 7), it can be difficult to determine specific associations between various personalized TCV components and any resulting clinical response (step 8). Illustrated findings are hypothetical and do not represent actual clinical trial data. CR, complete response; D, dose level; ELISPOT, enzyme-linked immunospot assay; LNK, linker; MHC, major histocompatibility complex; Neo, neoantigen; PD, pharmacodynamics; PK, pharmacokinetics; PR, partial response; SD, stable disease; UTR, untranslated region.